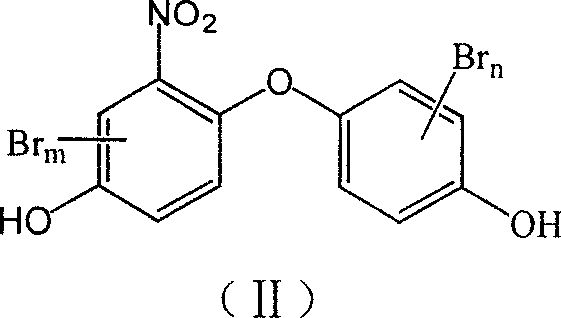
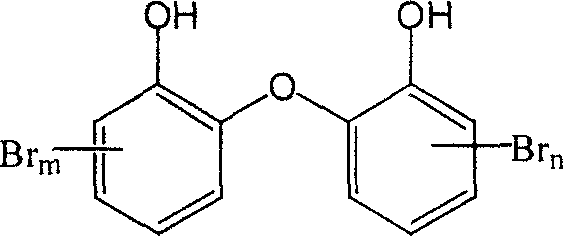
Bromo nitryl dihydroxy diphenyl ether compound and its synthesis method
A technology of bromonitrodihydroxydiphenyl ether and nitrodihydroxydiphenyl ether, which is applied in the field of bromonitrodihydroxydiphenyl ether compounds and their synthesis, can solve the problem of unsatisfactory antibacterial activity, Harm and other issues, to achieve the effect of reducing dosage and side effects, low cost, and safety of people and the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of intermediate 2-nitro-4,4'-bisacetoxydiphenyl ether
[0033] Add 2.54g (0.0126mol) 4,4′-dihydroxydiphenyl ether, 4.84g (0.04674mol) acetic anhydride (≥98.5%) into a 100mL three-necked flask with a magnetic stirrer, thermometer and reflux condenser. , Heat to reflux, monitor the reaction progress by TLC, after the reaction, cool the solution to room temperature, then add 4.85g (0.0468mol) acetic anhydride (≥98.5%) to the solution, stir well, slowly add 2.44g (0.0252mol) ) Concentrated HNO 3 (65%), react at room temperature, check the end of the reaction by TLC, after the reaction, use saturated NaHCO 3 Adjust the pH of the solution to 5-6, then extract the aqueous solution with ethyl acetate (3×20ml), separate the organic layer, and use anhydrous CaCl 2 After drying overnight, filtering, and removing the solvent under reduced pressure, a brown granular substance was obtained with a yield of 82.0%. Example 2: Synthesis of intermediate 2-nitro-4,4'-dihyd...
Embodiment 2
[0034] Add 4.17g (0.0126mol) 2-nitro-4,4'-bisacetoxydiphenyl ether, 30ml of 20% NaOH solution, 3ml into a 100mL three-necked flask equipped with a magnetic stirrer, thermometer and reflux condenser Ethanol, heat to reflux, monitor the reaction progress by TLC, after the reaction, adjust the pH to 1-3 with concentrated HCl, then extract the aqueous solution with ethyl acetate (3×20ml), separate the organic layer, and dry overnight with anhydrous sodium sulfate , Filtered, and the solvent was removed under reduced pressure to obtain a dark brown granular crude product. The crude product was separated by column chromatography to obtain a light brown solid material with a yield of 90.0%.
Embodiment 3
[0035] Example 3: Synthesis of target compound 3-bromo-2'-nitro-4,4'-dihydroxydiphenyl ether and detection of biological activity
[0036] Add 0.50g (0.0020mol) 2-nitro-4,4′-dihydroxydiphenyl ether and 2.64g (0.04mol / L) ice into a 100mL three-necked flask with a magnetic stirrer, thermometer and reflux condenser. Acetic acid, heat to reflux, dissolve 0.37g (0.0023mol) liquid bromine in 2.64g (0.04mol / L) glacial acetic acid, slowly drip into a three-necked flask, and react under reflux. TLC detects the end of the reaction, and the reaction until the red color basically fades , Cool to room temperature, use NaHCO 3 Adjust pH5-6, with 3%~5%Na 2 S 2 O 3 Wash with an aqueous solution to remove unreacted bromine, dry, evaporate the solvent, and recrystallize the crude product with petroleum ether to obtain the light yellow crystal target with a yield of 75.0%; 1 HNMR(CDCl 3, 400MHz) δ: 5.343 (s, 1H, OH), 5.512 (s, 1H, OH), 6.919 (dd, J=8.8, 2.8Hz, 1H, ArH), 6.970 (d, J=8.8Hz, 1H, ArH), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com