Preparation process of sertaconazole nitrate as antifungal medicine
A technology of sertaconazole nitrate and antifungal drug, which is applied in the field of preparation of antifungal drug sertaconazole nitrate, can solve problems such as high price, increase synthesis cost, complicated operation, etc. Cost, mild effect of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
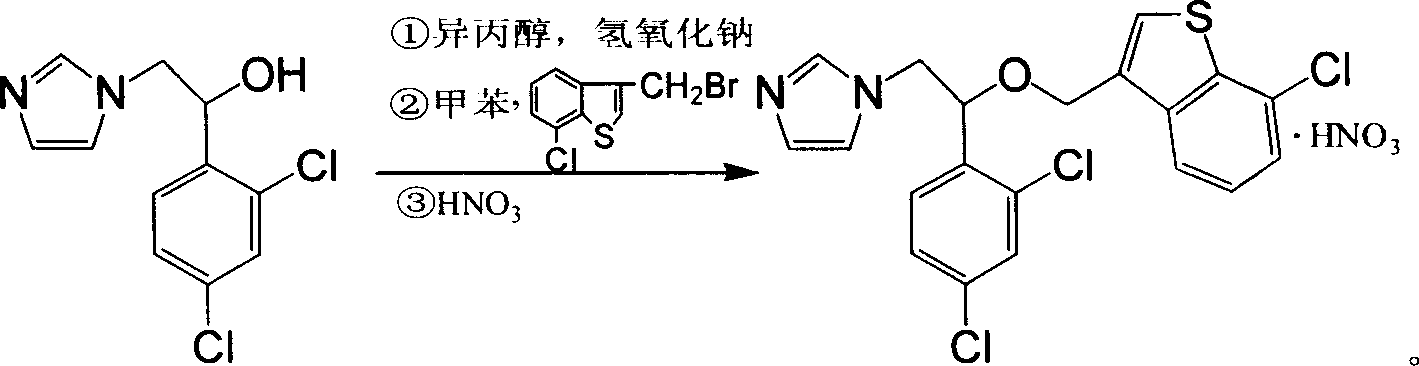
[0008] In the present invention, alcohol is mixed with 1-(2,4-dichlorobenzene)-2-(1-imidazole)ethanol, heated to reflux in the presence of sodium hydroxide, cooled, and 3-bromomethyl-7-chlorophenylpropane is added [b] Toluene solution of thiophene, stirred at room temperature, added water and organic solvent, allowed to stand still to separate the water layer, then added nitric acid, adjusted to pH 1-2, filtered, dried, and recrystallized to obtain sertaconazole nitrate.
[0009] Said every 25.6g (0.1mol) of 1-(2,4-dichlorobenzene)-2-(1-imidazole)ethanol and 26.0g (0.1mol) of 3-bromomethyl-7-chlorobenzo [b] Alcohol for thiophene: 120-150ml; organic solvent: 70-100ml. Said alcohol is isopropanol. Said organic solvent is one of toluene, cyclohexane or tetrahydrofuran. Said reflux is for 3-5 hours, then react at room temperature for 12-15 hours. Said alkali is sodium hydroxide or potassium hydroxide.
[0010] Embodiments of the invention:
[0011] Put 4.0g of sodium hydroxid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com