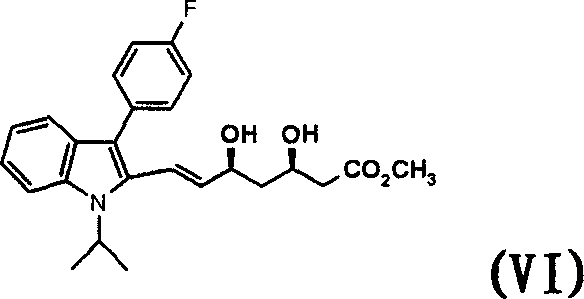
Method for preparing (3R, %S)-Fluvastatin
A technology of fluvastatin and fluorophenyl, which is applied in the production of bulk chemicals and organic chemistry, and can solve the problems of high catalyst price, great impact on the environment, and no industrial value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
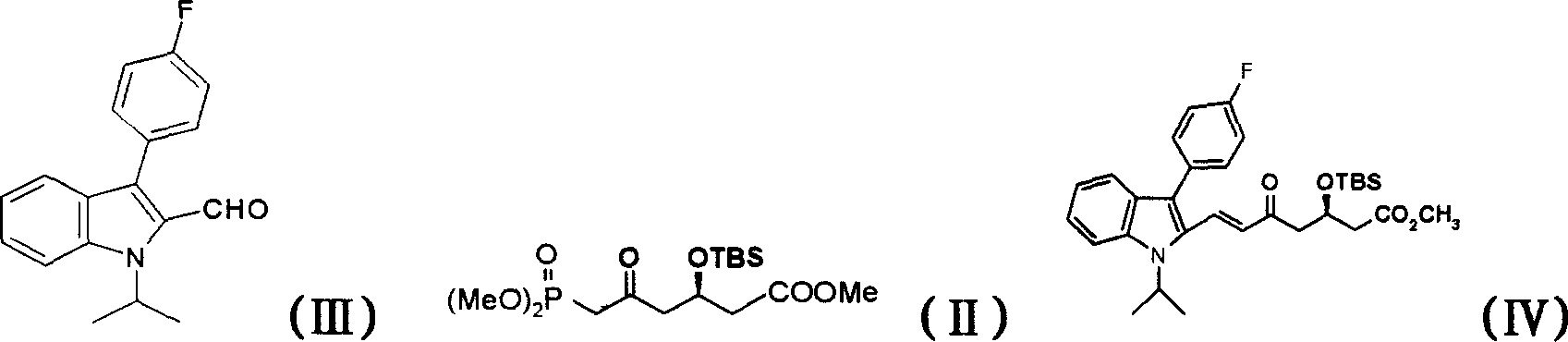
[0019] Example 1 (+)-(E)-7-[3'-(4"-fluorophenyl)-1'-(1"-methylethyl)indol-2'-yl]-5-carbonyl - Preparation of 3-tert-butyldimethylsilyloxy-6-heptenoic acid methyl ester (IV)
[0020] At room temperature, under the protection of nitrogen, add 2.3g of chiral side chain (II), 15ml of absolute ethanol, and 832mg of potassium carbonate to the reaction flask in sequence, stir for 25 minutes, then add 1.52g of parent nucleus aldehyde (III), and stir for about 48 hours at room temperature (TLC tracking, hexane:ethyl acetate=9.5:2), add 40ml of ethyl acetate, 20ml of water and stir, separate the organic layer, wash the ethyl acetate layer twice with water, once with saturated sodium chloride, and concentrate to dryness to obtain 3.2 g of crude orange-red oil was separated by silica gel column chromatography, eluting with petroleum ether: ethyl acetate = 10:1 to give 1.8 g of orange-red oil
[0021] 1H-NMR (CDCl3, 400NHz): δ0.02(s, 3H), 0.06(S, 3H), 0.8(s, 9H), 1.7(d, 6H), 2.5(m, 2H), 2...
Embodiment 2
[0024] Example 2 (+)-(E)-7-[3'-(4"-fluorophenyl)-1'-(1"-methylethyl)indol-2'-yl]-5-carbonyl - Preparation of 3-tert-butyldimethylsilyloxy-6-heptenoic acid methyl ester (IV)
[0025] At room temperature, under the protection of nitrogen, add 33.0g of chiral side chain (II), 200ml of isopropanol, 12.0g of potassium carbonate, 14.9g of parent nucleus aldehyde (III) to the reaction bottle, stir and react at room temperature for about 45 hours, then add 600ml of water , extracted twice with ethyl acetate 300ml and 100ml, separated the organic layer, washed the ethyl acetate layer twice with 500ml water, once with saturated sodium chloride, concentrated and dried to obtain 40.0g of crude red oil (IV).
Embodiment 3
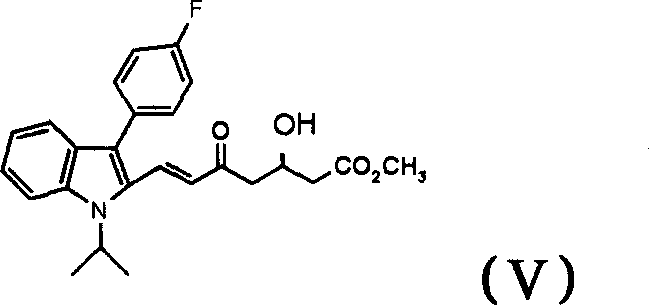
[0026] Example 3 (+)-(E)-7-[3'-(4"-fluorophenyl)-1'-(1"-methylethyl)indol-2'-yl]-3-hydroxy - Preparation of 5-oxo-6-heptenoic acid methyl ester (V)
[0027] At room temperature, under the protection of nitrogen, add 0.6g of the product of the previous step, 5ml of acetocyanide, and 0.4ml of 40% hydrofluoric acid to the reaction bottle and stir for about 1 hour (TLC tracking, hexane:ethyl acetate=10:3), add Ethyl acetate 30ml, washed with saturated sodium bicarbonate 2×30ml, washed with water, washed once with saturated sodium chloride, dried over magnesium sulfate, and the organic layer was concentrated to dryness to obtain 0.6g of crude orange-red oil, separated by silica gel column chromatography, hexane: Elution with ethyl acetate=6:4 gave 0.4 g of light yellow solid.
[0028] 1H-NMR (CDCl3, 400MHz): δ1.7(d, 6H), 2.5(m, 2H), 2.7(d, 2H), 3.6(s, 3H), 4.5(m, 1H), 4.9(m, 1H), 6.2(d, 1H), 7.1(m, 1H), 7.2(m, 2H), 7.3(m, 1H), 7.4(m, 2H), 7.5(d, 1H), 7.6(d, 1H ), 7.7(d, 1H)
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com