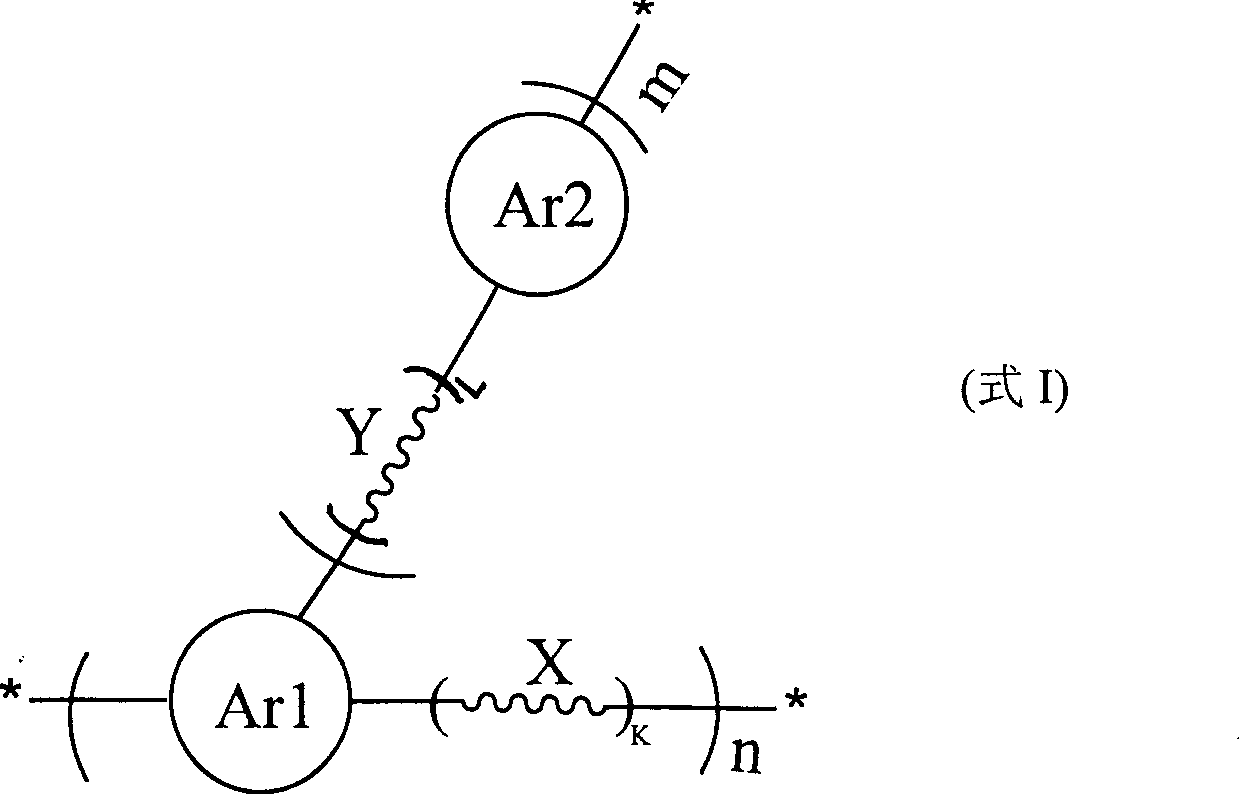
Two-dimensional conjugated polymer and its preparing method and application
A technology of conjugated polymers and polymers, applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve problems such as solar energy waste, reduce energy gap, broaden absorption spectrum, and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
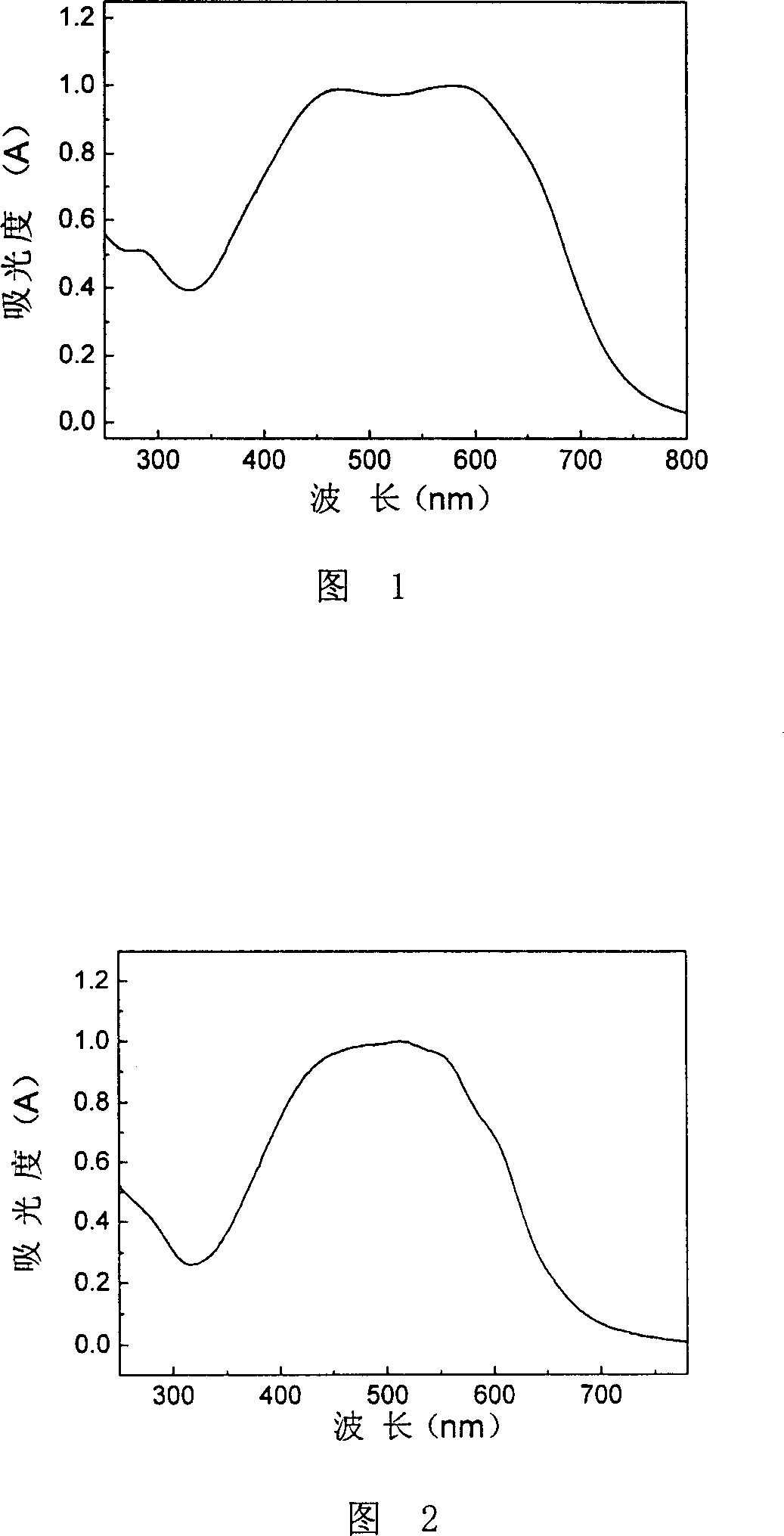
[0036] Embodiment 1, prepare the conjugated polymer of formula II structure
[0037] The conjugated polymer of formula II structure has 2-(E)-((2-((E)-2-(5-alkylthiophen-2-yl) vinyl) thiophen-2-yl) vinyl side Chain thiophene ethylene and 3-alkyl thiophene ethylene binary copolymer, its preparation reaction formula is as follows:
[0038]
[0039] The specific description is as follows:
[0040] Step 1) Mix 0.1 mol of 2-chloromethylthiophene with 0.1 mol of trimethyl phosphite, heat and reflux to 170°C, react for one hour and then cool down, add N,N'-dimethylformamide (DMF for short) at room temperature ) 50 milliliters, add sodium methoxide 0.1mol, after stirring for 10 minutes, add 5-dodecyl-2-thiophene carboxaldehyde 0.1mol, after stirring for 30 minutes, extract, isolate 2-dodecyl-5-(( E)-2-(thiophen-2-yl)vinyl)thiophene.
[0041]Step 2) Under nitrogen protection, 0.1 mol of 2-dodecyl-5-((E)-2-(thiophen-2-yl) vinyl)thiophene is mixed with 100 ml of tetrahydrofuran, an...
Embodiment 2
[0046] Embodiment 2, prepare the conjugated polymer of formula III structure
[0047] The conjugated polymer of formula III structure has 2-(E)-((2-((E)-2-(5-alkylthiophen-2-yl) vinyl) thiophen-2-yl) vinyl Terpolymer of thiophene and 3-alkylthiophene and thiophene, its preparation reaction formula is as follows:
[0048]
[0049] After mixing 0.1 mol of thiophene with 100 ml of tetrahydrofuran, add 0.2 mol of butyllithium under nitrogen protection, reflux for 2 hours, add 0.25 mol of tributyltin chloride, stir for 12 hours, and distill under reduced pressure to obtain 2, 5-Bis(tributyltinyl)thiophene. 2,5-bis(tributyltin-based)thiophene 1mmol, 2,5-dibromo-3-(2-(E)-((2-((E)-2-(5-dodecylthiophene- 2-yl) vinyl) thiophen-2-yl) vinyl) thiophene 1mmol (prepared in Example 1), 2,5-dibromo-3-hexylthiophene 0.5mmol, tetrakis (triphenylphosphine) palladium 0.02mmol, 10ml of toluene, mix well in the flask, under the protection of nitrogen, heat up to 80-100°C, react for 12-24 hours...
Embodiment 3
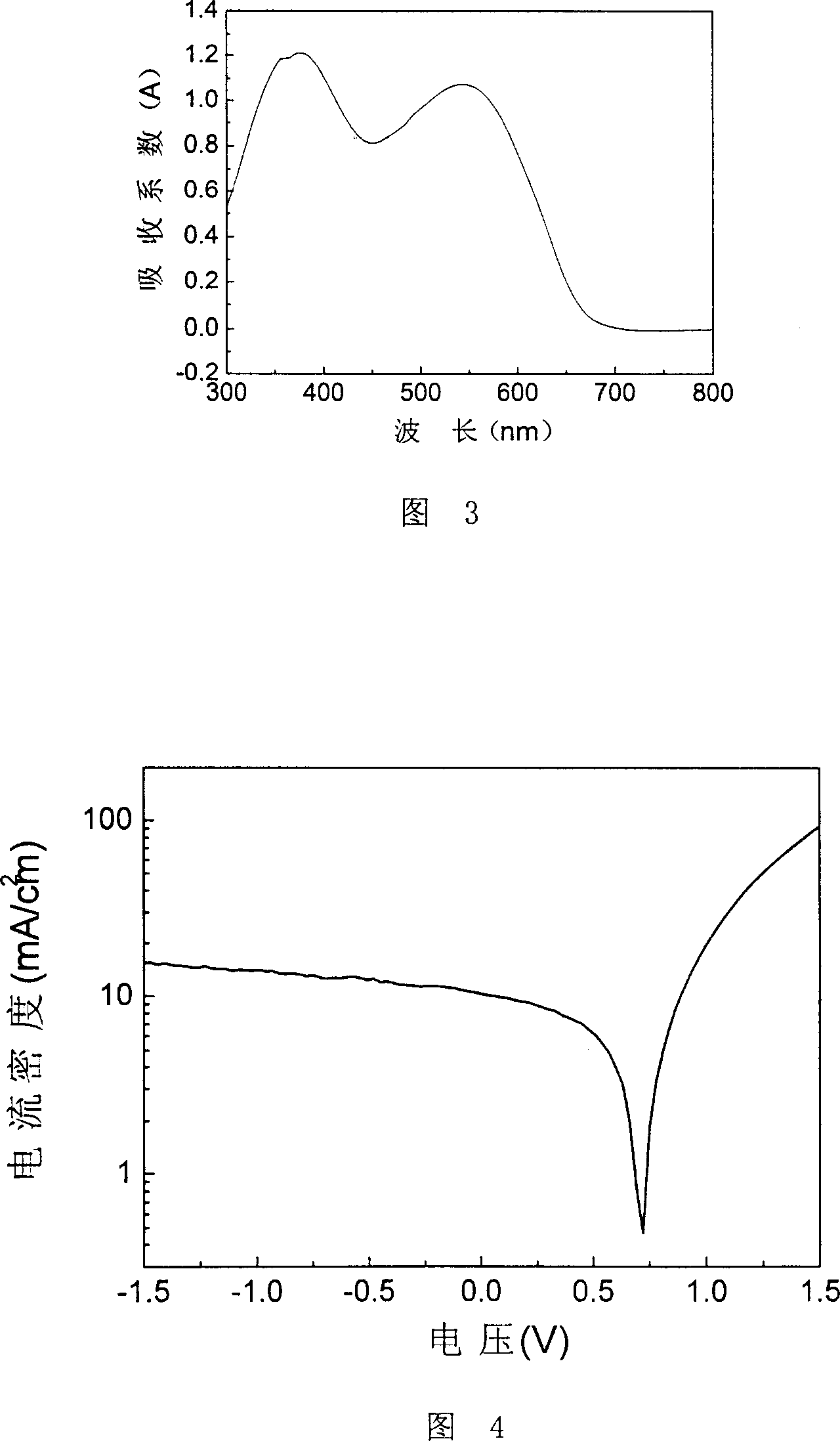
[0052] Embodiment 3, prepare the conjugated polymer of formula IV structure
[0053] The conjugated polymer of formula IV structure is thiophene with 2-(E)-(4-(2-(E)-(4-alkoxyphenyl) vinyl) phenyl) vinyl side chain and 3- Terpolymer of alkylthiophene and thiophene.
[0054]
[0055] Step 1) Mix 2,5-dibromo-3-bromomethylthiophene with 0.1 mol of trimethyl phosphite, heat to reflux to 170°C, react for one hour and then cool down, then add N,N'-dimethyl at room temperature Formamide (abbreviated as DMF) 50 ml, add 0.1 mol of sodium methoxide, stir for 10 minutes, add 0.1 mol of p-tolualdehyde, stir for 30 minutes, extract, recrystallize to separate 3-(2-(E)-( 4-methylstyryl))-2,5-dibromothiophene.
[0056] Step 2) Mix 3-(2-(E)-(4-methylstyryl))-2,5-dibromothiophene and bromosuccinamide with 0.1 mol each, add 50 ml of carbon tetrachloride, 500 watts Reflux for 3 hours under ultraviolet light, recrystallize and separate 3-(2-(E)-(4-bromomethylstyryl))-2,5-dibromothiophene.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com