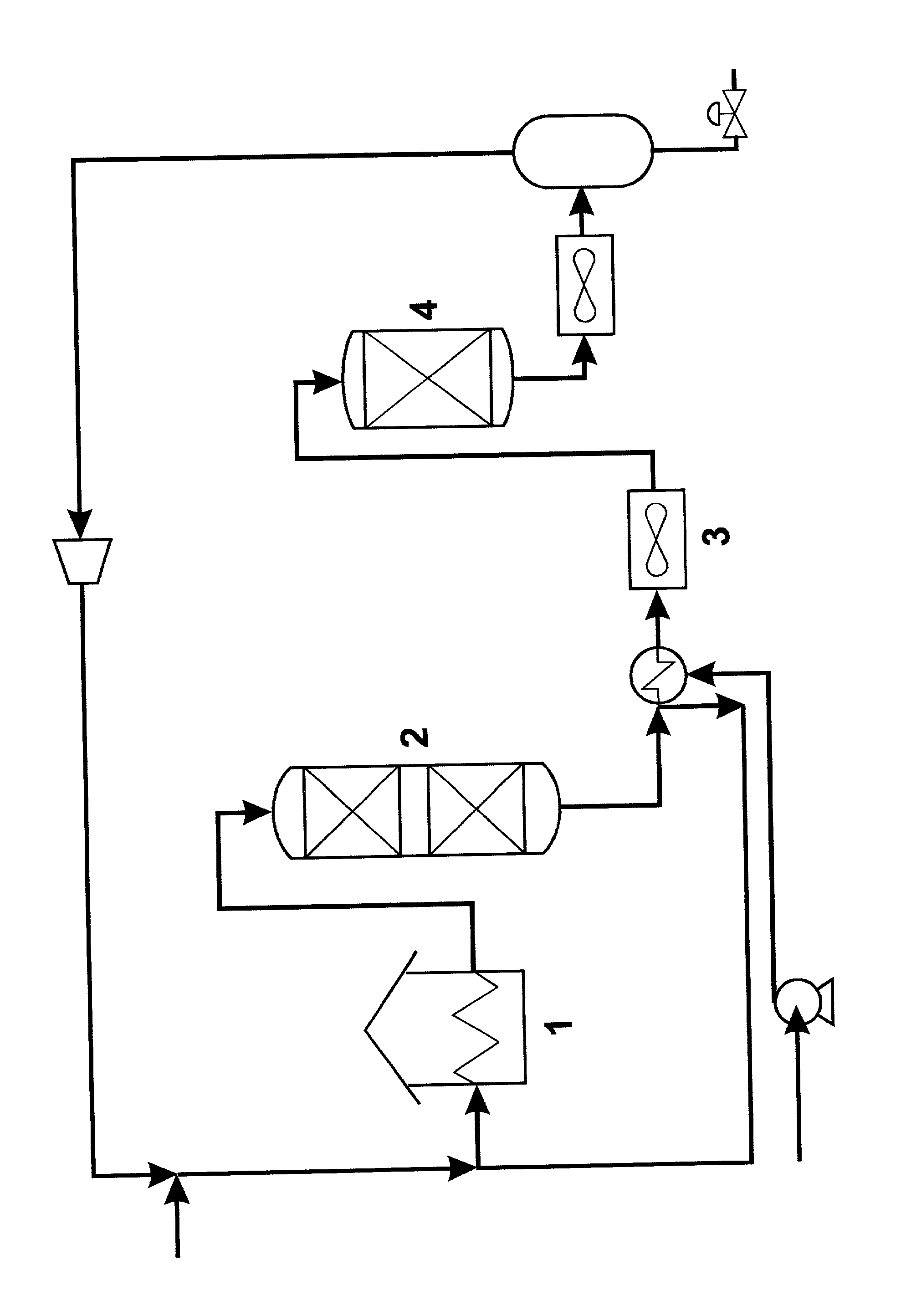
Process for reducing content of sulphur compounds and poly-aromatic hydrocarbons in a hydrocarbon feed
a technology of polyaromatic hydrocarbons and hydrocarbon feeds, which is applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, fuels, etc., can solve the problems of increasing the catalyst's coke-making propensity, reducing the yield of valuable products, and high costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0035] Product A from Example 1 is further hydrotreated at lower temperatures at high LHSV. The pressure is 50 bar, which is identical to the pressure at which product A was obtained.
[0036] A Ni--Mo on alumina catalyst is used in this test. The results are shown in Table 3.
3TABLE 3 Properties of products in Example 2: Temp-era-N Di-aro- Tri-ture LHSV SG S (wt matics aro- PAH (.degree. C.) (h.sup.-1) 60 / 60 (wt %) ppm) (wt %) matics (wt %) 325 6 0.8914 0.0038 505 4.6 5.0 9.6 350 6 0.8911 0.0029 468 4.9 4.9 9.5
[0037] As illustrated from the Table 3 there are a remarkable sulphur and nitrogen removal in this low temperature hydrotreatment, and further it is quite obvious that a large amount of the PAH can be removed at a relatively high LHSV during this low temperature posttreatment. Both the sulphur removal and the PAH removal is due to the shift in equilibrium.
example 3
[0038] Product B from Example 1 is further hydrotreated at lower temperatures at different LHSV and temperatures. The pressure is 50 bar, which is identical to the pressure at which product B was obtained. A Ni--Mo on alumina catalyst is also used in this test. The results are shown in Table 4.
4TABLE 4 Properties of products in Example 3: Tem- Tri-pera-N Di-aro- aro-ture LHSV SG S (wt matics matics PAH (.degree. C.) (h.sup.-1) 60 / 60 (wt %) ppm) (wt %) (wt %) (wt %) 300 2 0,9369 0,1500 2058 7,7 20,1 27,8 300 4 0,9390 0,1588 2067 10,2 21,3 31,5 300 6 0,9406 0,1618 2080 9,9 21,4 31,6 350 2 0,9335 0,1049 1657 6,6 17,0 23,6 350 4 0,9365 0,1317 1870 9,2 18,1 27,3 350 6 0,9378 0,1442 1877 9,6 19,3 28,9
[0039] Again it is clear that a large amount of the poly-aromatic compounds can be removed at low temperature (and the same pressure) due to the shift in equilibrium. Again there is a significant and important sulphur removal at this low-temperature hydrotreatment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| outlet temperature | aaaaa | aaaaa |
| outlet temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com