Photosensitizers with ligand targeting properties for tumor therapy
a technology of ligand targeting and tumor therapy, applied in the direction of drug compositions, peptide/protein ingredients, peptide sources, etc., can solve the problems of reducing the usefulness of the drug, the potential of being more selective but not less effective, and several problems, so as to reduce the therapeutic dose of the drug and reduce the side effects of the therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
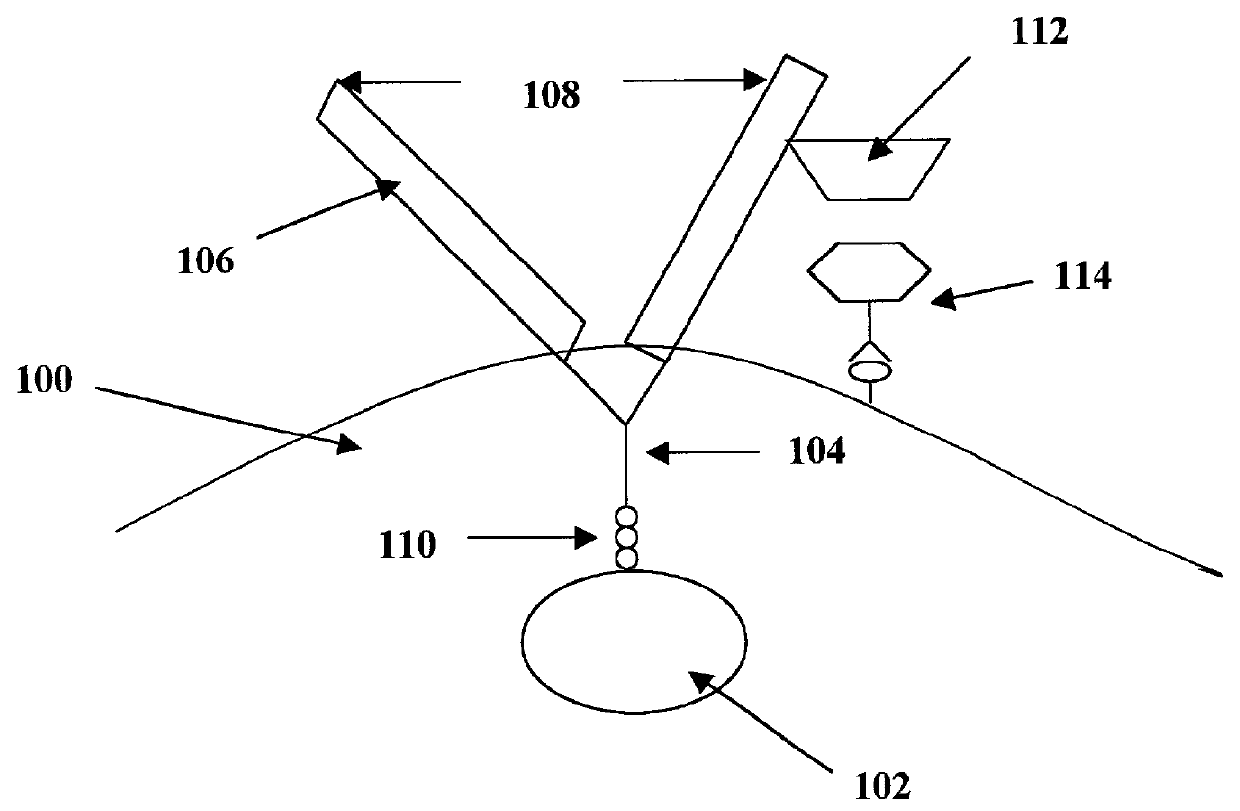
Image
Examples
example 2
Construction of a Cyclodextrin Parachute with Therapeutic Compound
[0034] a. OH-Bacteriopheophorbide is prepared in the usual manner, slightly acidified, and reacted first with an equimolar amount of di-isopropyl carbodi-imide. After half an hour N-hydroxy succinimide is added in equimolar amount and reacted over night.
[0035] b. Hydroxypropyl-.gamma.-cyclodextrin (Molecusol.TM.) is dissolved in pyridine and reacted with toluene sulfonyl chloride in triple-molar amount. The mixture reacts for 3 hours at room temperature and is freeze-dried. The product is dissolved in water containing 10 fold excess of diamino propane. After incubation overnight the product is freeze-dried again.
[0036] c. Products from, A and # B are mixed in equimolar amounts. Finally, biotin-N-hydroxy succinimide is added in sufficient excess (more than 2 mole equivalents) to obtain the desired product: di-biotinyl hydroxy bacteriopheophorbide amidopropylamide gamma-cyclodextrin.
example 3
Treatment of Patients with the Substance and PDT
[0037] For a patient, a dose of 0.8-1.0 mg / kg of the product should be sufficient for intravenous injection. Irradiation should follow at 760 nm and after only 15 min. The light power should be reduced to 200 mW, and no more than 20 J / cm.sup.2 should be applied. The persistence of the dye in blood can be followed by fluorescence spectrometry and a reduction by a factor of 10 should be observed after 1 week.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hydrophilic | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com