Paramagnetic polymerized protein microspheres and methods of preparation thereof
a polymerized protein and microsphere technology, applied in the field of contrast agents, can solve the problems of reducing the efficacy of contrast agents, restricting the use of particular contrast agents to one imaging mode, complex interaction between encapsulated microbubble contrast agents and ultrasound, etc., and achieve the effect of accurate simulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ultrasound Studies
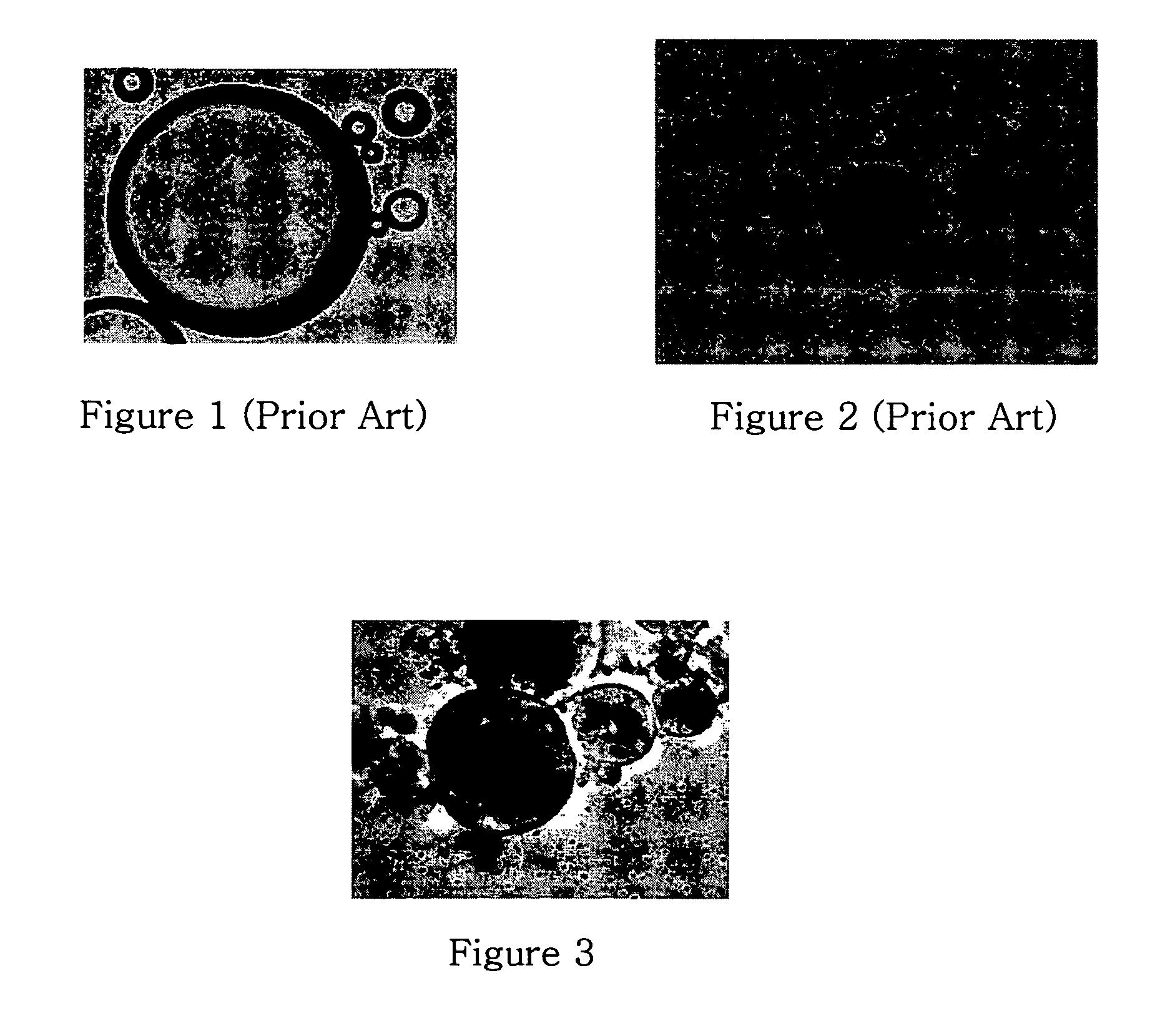
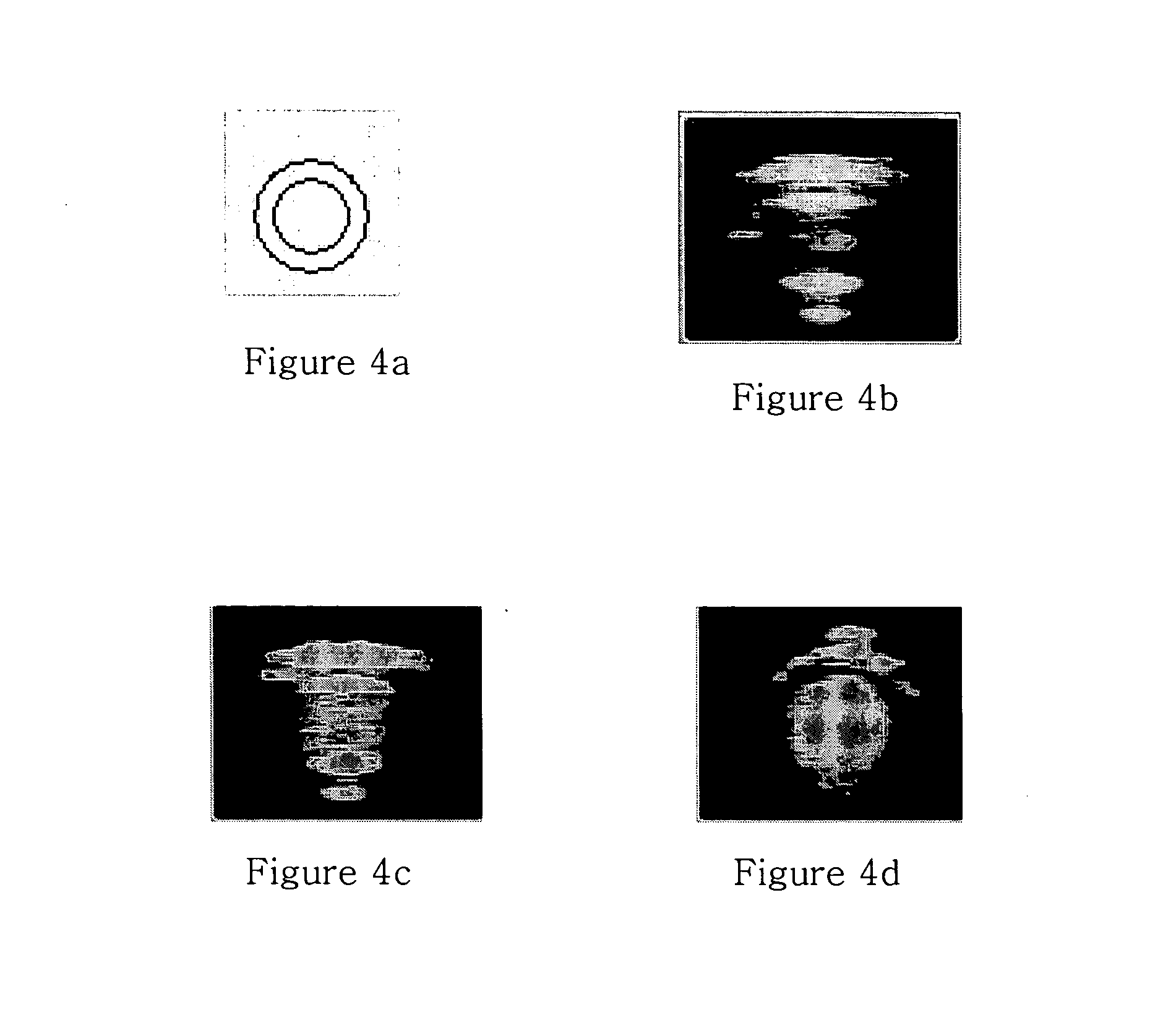
Preliminary imaging studies were conducted to compare the cross-sectional ultrasound images of oil (containing no contrast agents), air-filled albumin microspheres, and unmodified and surface-modified GOAM flowing through a tube. An Aloka SSD5500 PHD ultrasound machine with a linear transducer (UST 5539 10 MHz) was used to create traditional B-mode ultrasound images. In these experiments, the above solutions were injected into clear plastic Tygon tubing (OD=0.318 cm; ID=0.159 cm) (FIG. 4a) immersed in degassed water at room temperature. Cross-sectional images of the tube were captured using a personal computer, video frame grabber and real time video capture software (Capture©, Watkin, 1997). These images are shown in FIGS. 4b-4d.
These images clearly demonstrate the full circumference visualization capabilities of the various media—oil (no contrast media), air-filled albumin microspheres, and GOAM. Differences are clearly evident in the cross-sectional B-mode u...
example 2
Physical Characterization of Unmodified and Surface-Modified GOAM
The determination of the size distribution, concentration, and size fractionation of the synthesized GOAM is accomplished via Coulter counter analysis. In addition, optical microscopic images (Bausch & Lomb) are used to verify the size and conformation of GOAM.
example 3
Acoustic Simulations
Two different simulation approaches are required to describe the characteristics of the acoustic driving forces on the developed microsphere as well as the acoustic propagation of the scattered ultrasonic energy. One approach uses boundary element method (BEM) modeling to describe the acoustic behavior of the microsphere. Finite difference time domain modeling (FDTD) is used to examine the backscattering properties of the reflected acoustic pressure waves.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molar concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com