Redox active reversible electrode and novel battery using the same
a reversible electrode and redox technology, applied in cell components, electrochemical generators, transportation and packaging, etc., can solve the problems of slow electron transfer reaction, faradaic current response based on redox reaction cannot be obtained at a certain constant potential, and film made of this material has no electron conductivity, etc., to achieve high energy density, high electron conductivity, and high electron transfer promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0063] An acetonitrile (AN) solution (solution for electrolytic polymerization) containing 20 mM of EDOT monomer among the thiophene compounds represented by the formula (II) and 0.1M of lithium perchlorate (LiClO4) as a supporting electrolyte was prepared.
[0064] A PEDOT coated electrode was prepared in the following manner. Using a 3-electrode type cell, with a glassy carbon disk electrode having a diameter of 3 mm used as a working electrode, a coil platinum wire used as a counter electrode and a silver ion electrode used as a reference electrode, an electrolytic oxidative polymerization was carried out in the solution for electrolytic polymerization described above, thereby preparing a PEDOT coated electrode. The silver ion electrode was prepared by dissolving 0.5M silver perchlorate into the solvent (AN) used, and employing a commercially available holder with the solvent used as an inner solution. The glassy carbon disk electrode was used after polishing it with polishing alum...
example 2
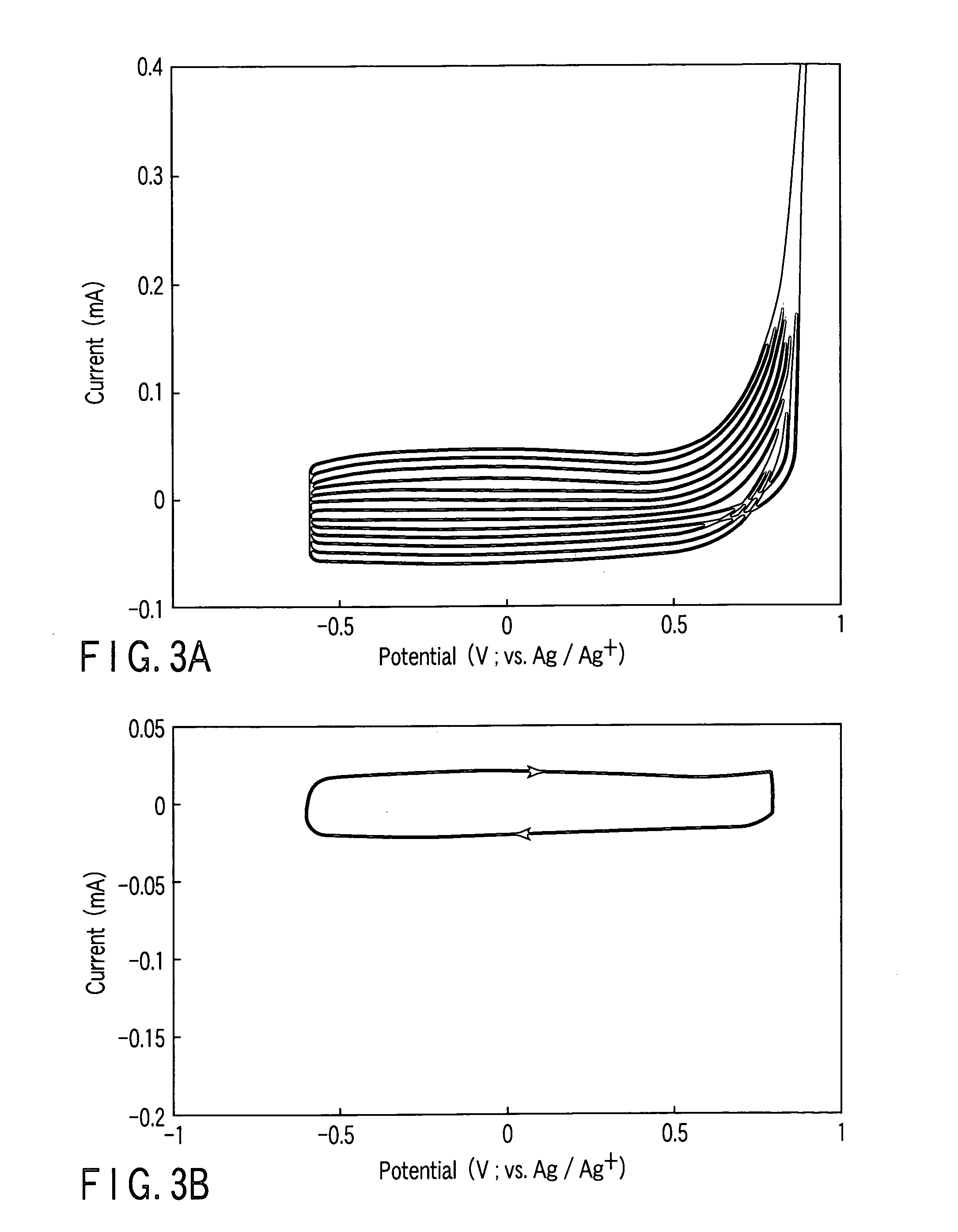
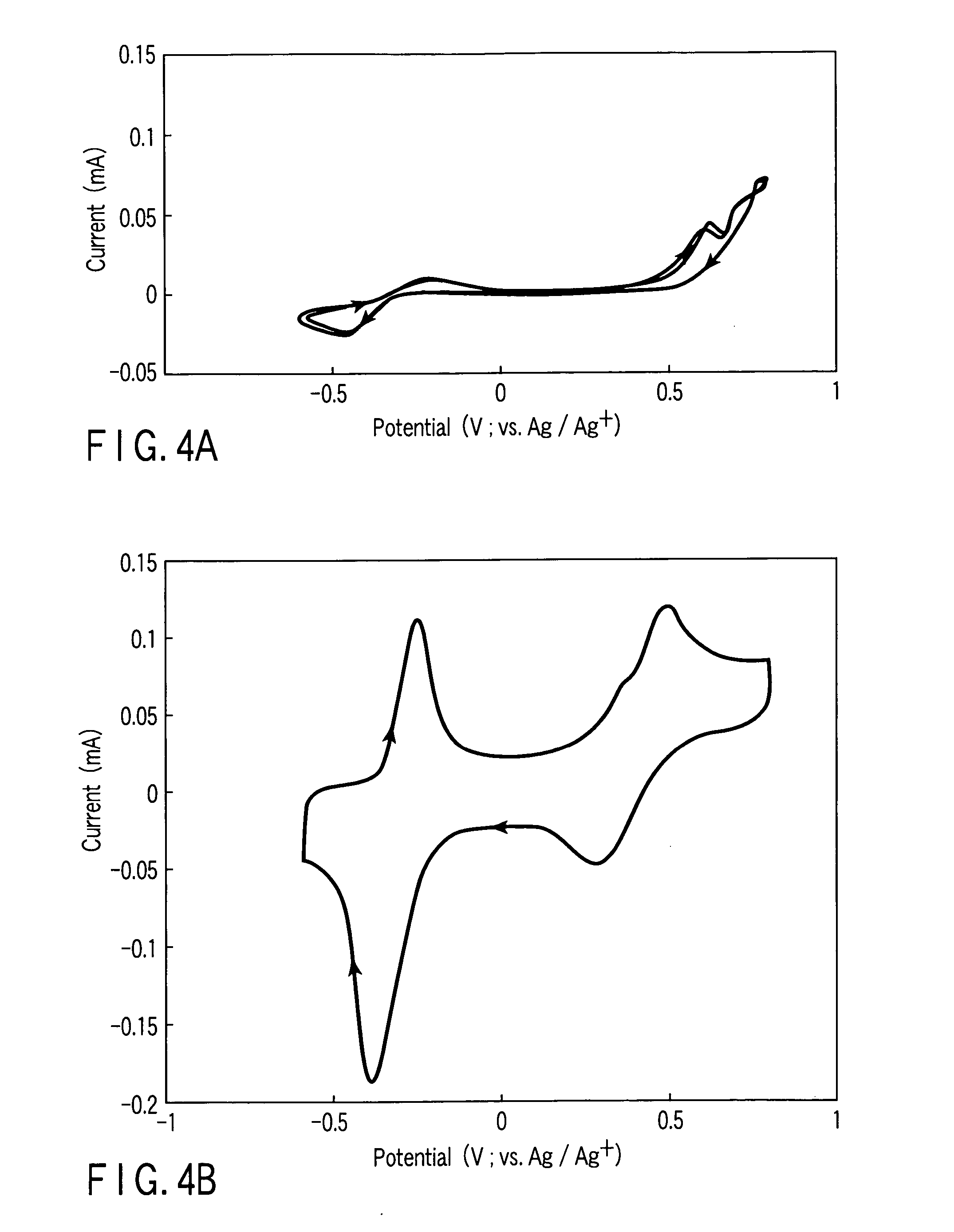
[0066] As a typical example of the organic sulfur compound, 2,5-dimercapto-1,3,4-thiadiazole (DMcT) was selected. 1.0M LiBF4 was dissolved into an AN solution of 1.0M LiClO4, an N-methyl-2-pyrrolidinone (NMP) solution of 1.0M LiClO4, and a solution obtained by mixing propylenecarbonate (PC) and ethylenecarbonate (EC) at a weight ratio of 1:1, each containing 5 mM of DMcT, to prepare an electrolytic solution. Subsequently, the glassy carbon electrode as the working electrode coated with the PEDOT film was prepared by the same method as the method of Example 1, using the electrolytic solutions prepared above. Then, the CV measurement was carried out.
[0067]FIGS. 4A and 4B are CVs of DMcT, which were measured using a PEDOT uncoated electrode and the PEDOT coated electrode, respectively, in the AN electrolyte solution. The measurements were carried out while changing the potential sweeping range. The CVs obtained by performing potential sweeping in a range of from −0.6V to +0.8V are sho...
example 3
[0070] A solution used for measurement was prepared by adding DMcT to an NMP containing 0.1M LiClO4 to make 2 mM DMcT solution. As the working electrode, a glassy carbon disk electrode (having a diameter of 3 mm) for the hydrodynamic voltammetry was used. In the same manner as that of Example 2, a PEDOT coated electrode was prepared. Using a coil platinum wire as the counter electrode and a silver ion electrode as the reference electrode, the measurements were carried out. FIG. 5 shows current-potential curves for the oxidation reaction of from a monomer to a dimmer of DMct obtained from a rotation speed of 400 rpm (number of rotation / min) at the PEDOT thin film and the uncoated electrode. The graph (a) in FIG. 5 is a current-potential curve obtained with use of the uncoated electrode. An increase in limiting current was observed as the rotation speed increased. Further, as the rotation speed increased, the half wave potential was shifted to the positive electrode side. The graph (b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com