Method for combined parallel agent delivery and electroporation for cell structures and thereof
a cell structure and electroporation technology, applied in the field of combined parallel agent delivery and electroporation for cell structures, can solve the problems of difficult transfer of all these compounds and particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
second embodiment
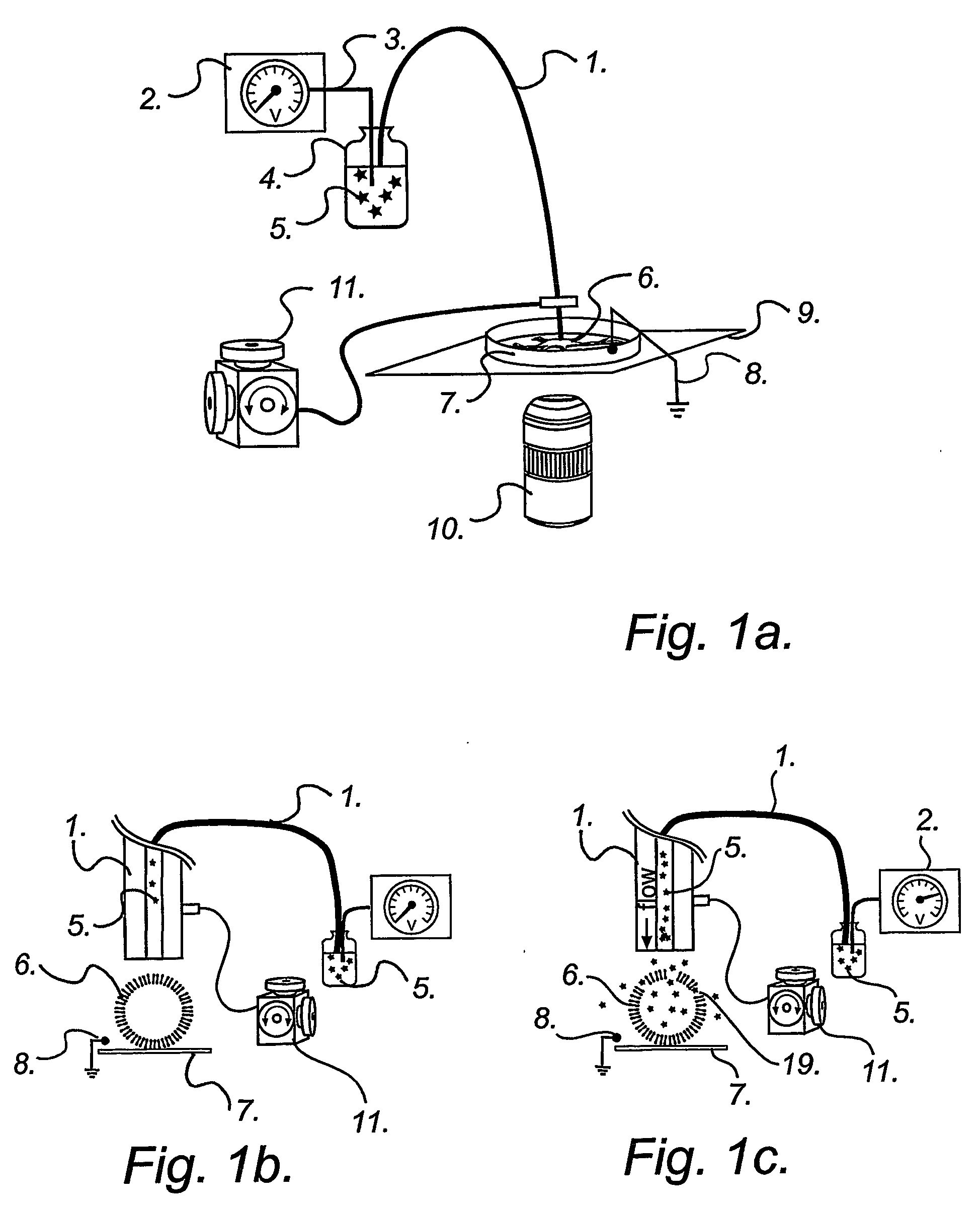
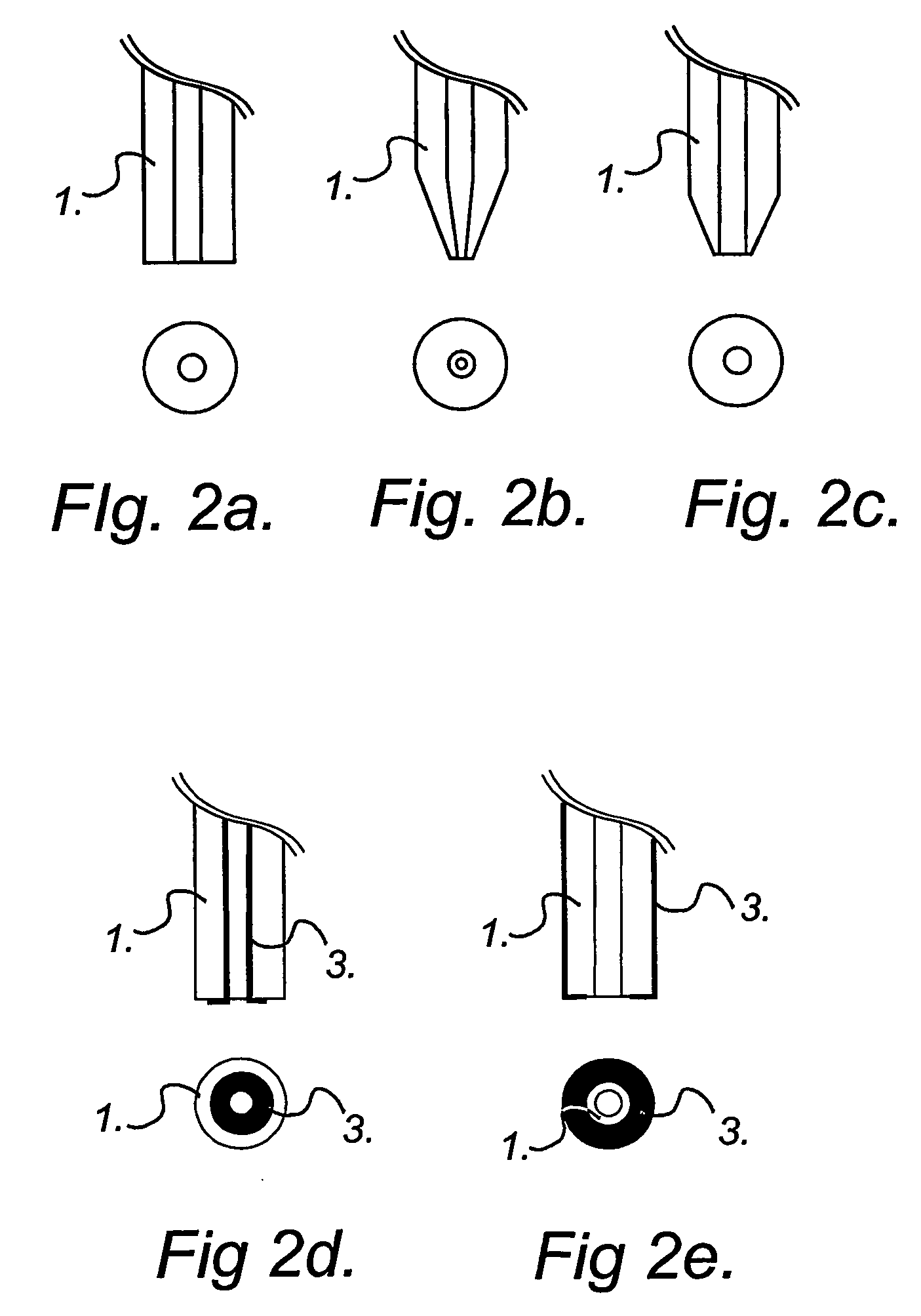
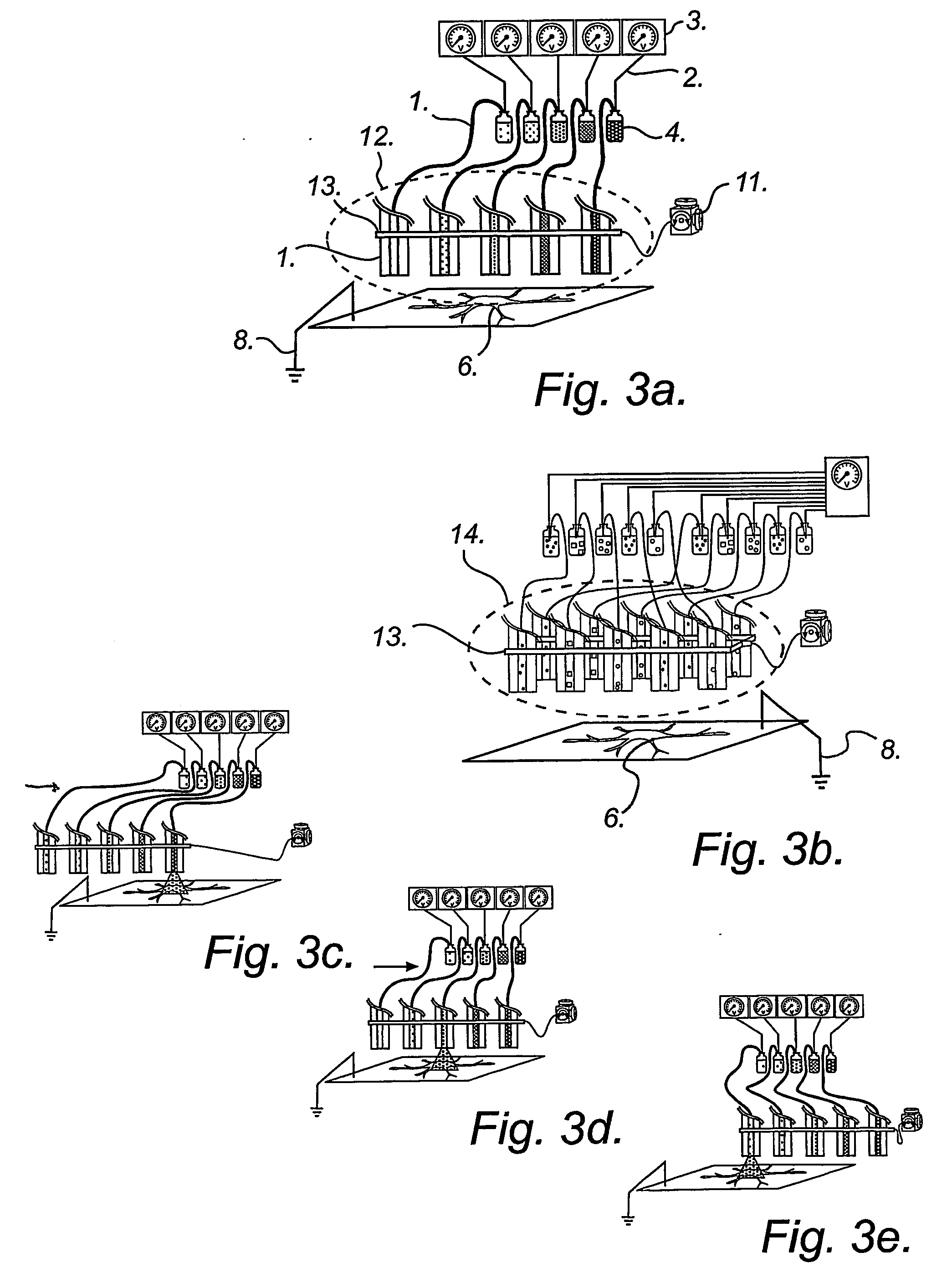
According to another embodiment, sequential delivery of multiple cell-loading loading agents is achieved by connecting a conventional EFC to a microfluidic switch for sample stacking. In this configuration a plurality of loading agents can be sequentially introduced into the EFC and subsequently be delivered to a single or several cellular targets. The cell-loading agents can be introduced in discrete zones in the EFC by any of the pumping modes discussed above. Either the EFC is preloaded with cell-loading agents before electroporation experiments or the EFC is loaded on-the fly, that is during electroporation experiments. An example of this second embodiment is illustrated in FIG. 5.
According to yet another embodiment, sequential delivery of multiple cell-loading agents is achieved by introducing a separation step while pumping the fluid through the EFC. When using electrophoretic pumping for delivery of reagents, all species present in the electrolyte solution is separated based...
example 1
Detection of the Intracellular Receptor Ryanodine Type II
Fluo-3 AM ester was from Molecular Probes (Leiden, The Netherlands). Cyclic ADP ribose and the chemicals used for buffer solutions, were all of analytical grade and purchased from Sigma (St. Louis, Mo., USA). All solutions were made in distilled water from a Milli-Q system (Millipore).
NG108-15 cells were plated on no. 1 cover slips or in a Petri dish and allowed to grow for 1-3 days. Cell dishes were mounted in a circular polycarboneat holder and transferred to the stage on the microscope. Prior to experiments the culture medium was replaced by a HEPES buffer (NaCl 140 mM, KCl 5.0 mM, MgCl2 1.0 mM, CaCl2 1.0 mM, D-glucose 10 mM, HEPES 10 mM, pH was adjusted to 7.4 with NaOH).
The cells were stained with fluo-3 AM ester by incubating the cells for 30 minutes in dye solution (10 μM fluo-3 AM ester in HEPES buffer) at room temperature. To remove excess uncaptured dye, the cells were washed three times in HEPES buffer and st...
example 2
Detection of Intracellular Enzymes I. Detection of Proteases
Casein BODIPY FL was obtained from Molecular Probes (Leiden, The Netherlands). The chemicals used for buffer solutions were all of analytical grade and purchased from Sigma (St. Louis, Mo., USA). All solutions were made in distilled water from a Milli-Q system (Millipore).
Cell culturing and preparations were made according to methods used in example 1 above, and apparatus and instrumentation was the same as in example 1.
Electroporation was performed as in example 1 and an EFC (30 cm long, 50 μm id., 375 μm o.d.) was used. Casein BODIPY FL was used in a concentration of 100 μg / ml and electroporated into cells using a 10 second pulse at 10 kV.
The results of detection of intracellular proteases using casein-BODIPY-FL is shown in FIG. 9. Specifically, the intracellular protease activity was investigated using a protein, casein, which was heavily loaded with the green-fluorescent molecule BODIPY FL, as enzyme substrate....
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| close distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com