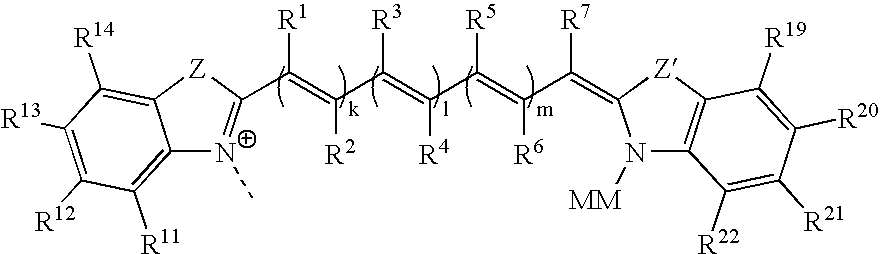
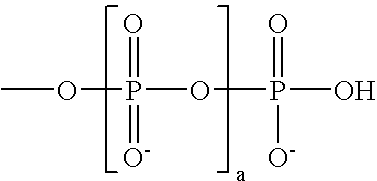
Mobility-modifying cyanine dyes
a technology of cyanine dye and cyanine, which is applied in the field of fluorescent dye compounds, can solve the problems of inability to rationally design such sets of “mobility-matched” terminators, and no method exists to and achieve high enzymatic activity and predictably alter electrophoretic mobilities of polynucleotides.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] 5.1 Abbreviations
[0030] The abbreviations used throughout the specification to refer to certain nucleobases, nucleosides and / or nucleotides are those commonly employed in the art and are as indicated below:
[0031] Expression Abbreviation [0032] adenine A [0033] 7-deazaadenine 7-deaza-A [0034] N6-Δ2-isopentenyladenine 6iA [0035] N6-Δ2-isopentenyl-2-methylthioadenine 2 ms6iA [0036] cytosine C [0037] guanine G [0038] 6-thioguanine 6sG [0039] 7-deazaguanine 7-deaza-G [0040] N2-dimethylguanine 2dmG [0041] 7-methylguanine 7mG [0042] thymine T [0043] 4-thiothymine 4sT [0044] uracil U [0045] dihydrouracil D
[0046] Expression Abbreviation [0047] 4-thiouracil 4sU [0048] base Y Y [0049] ribonucleoside-5′-triphosphate NTP [0050] adenosine-5′-triphosphate ATP [0051] 7-deazaadenosine-5′-triphosphate 7-deaza-ATP [0052] cytidine-5′-triphosphate CTP [0053] guanosine-5′-triphosphate GTP [0054] 7-deazaguanosine-5′-triphosphate 7-deaza-GTP [0055] thymidine-5′-triphosphate TTP [0056] uridine-5′-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com