Small organometallic probes
a technology of organometallic probes and probes, which is applied in the field of small organometallic probes, can solve the problems of low targeting of gold, affecting the stability of the probe,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Preparation of Fluorescent and Gold Immunoprobes
1. Preparation of Fluorescein-Conjugated Nanoqold Using Fluorescein-Pbosphine
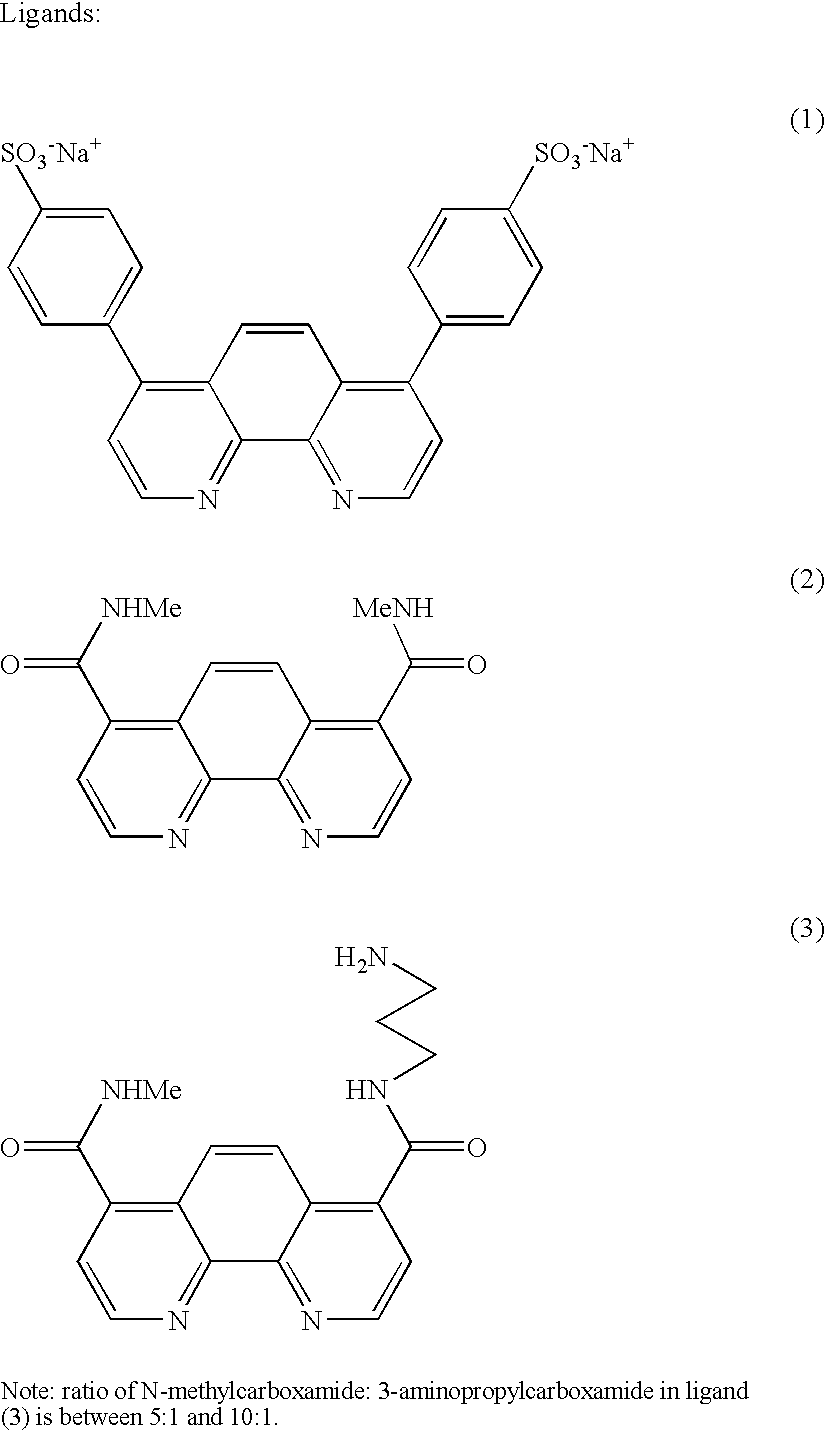
[0034] A tris (aryl) phosphine ligand bearing a single fluorescein substitutent, and a second tris (aryl) phosphine ligand bearing a single primary amine, were mixed with tris (p-N-methylcarboxamidophenyl) phosphine in the ratio 2:1:5. Ninety mg of this ligand mixture in 25 mL of methanol (an estimated twelve-fold molar excess) was added to a solution of freshly prepared Nanogold (product from 0.4 g of gold (I) triphenylphosphine chloride) in dichloromethane (25 mL) and stirred at room temperature overnight.
[0035] The reaction mixture was extracted with 0.02M ammonium acetate with acetic acid, pH 5.8, in 20% isopropanol / water (3×150 mL), then evaporated to dryness, redissolved in DMSO (2 mL) and 0.6M triethylammonium bicarbonate in 20% isopropanol / water. The fluorescein-substituted Nanogold was isolated by gel filtration, using a coarse gel in a large col...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com