Multidimensional pump apparatus and method for fully automated complex mixtures separation, identification, and quantification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
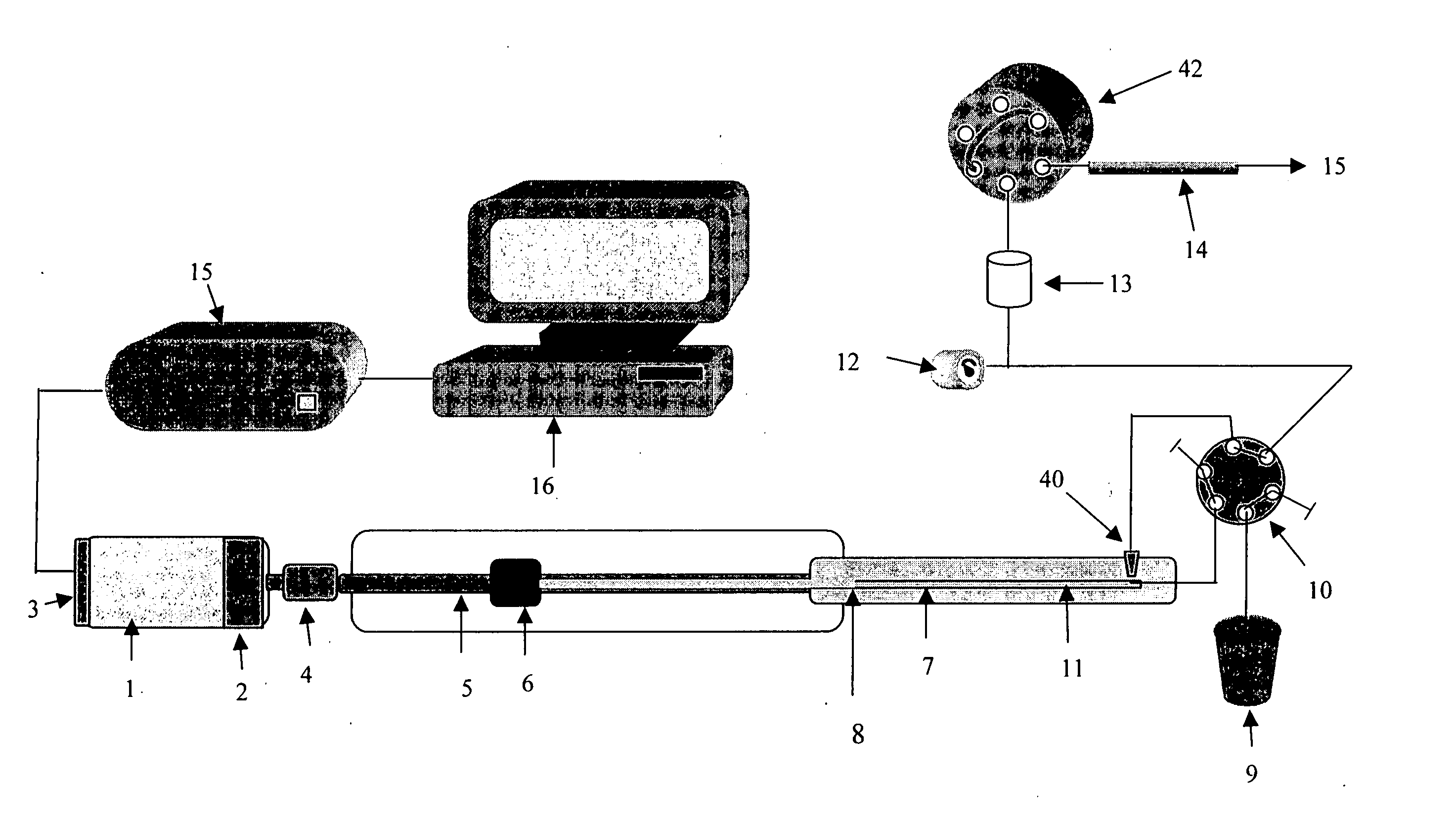
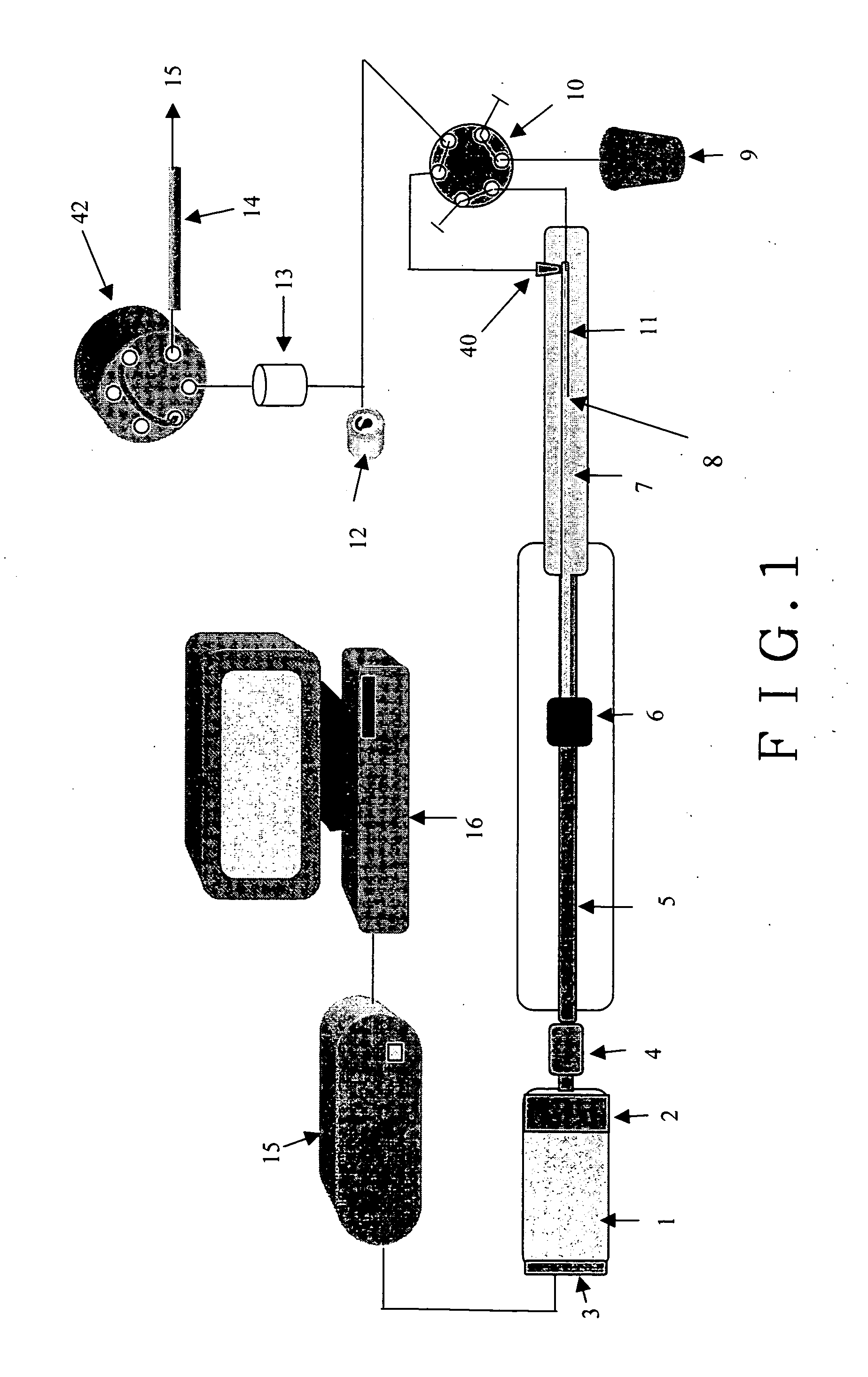
[0027]FIG. 1 is a simplified block diagram depicting key components of a preferred embodiment of a pumping system having a Servomotor 1 (or a step motor) with a gear box 2 of 50 to 1 ratio that has an optical encoder 3 mounted on the motor shaft for closed loop digital control for a motor 1. The motor 1 can be controlled to have one revolution in 40 to 80 minutes or longer. A total of 14,336,000 digital count is required to move the piston 7 linearly for 7 cm. The piston 7 is a 0,159 cm diameter zirconium oxide that has piston volume of 150 μl. The motion of the piston 7 is controlled by proportion, integration and differentiation (PID) algorithm to ensure reproducible 0.5 nl / digital count closed loop control. At 40 minutes per revolution or 1 mm travel length, the minimum flow rate can be deliver with the servo motor 1 alone is 50 nl / min. The preferred embodiment of the invention having also a gear box 2 of 50 to 1 speed reduction factor to further increase the control accuracy to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com