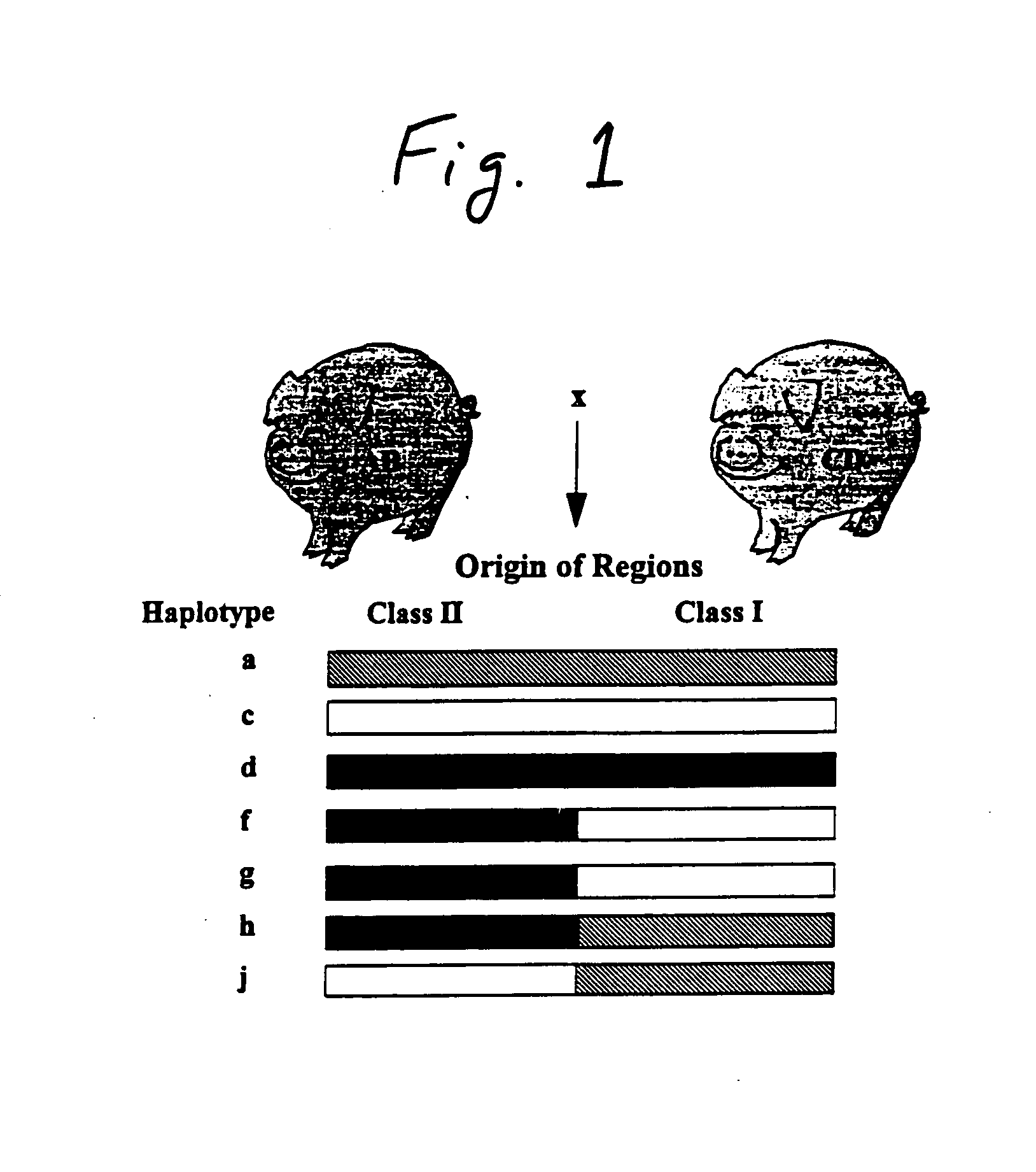
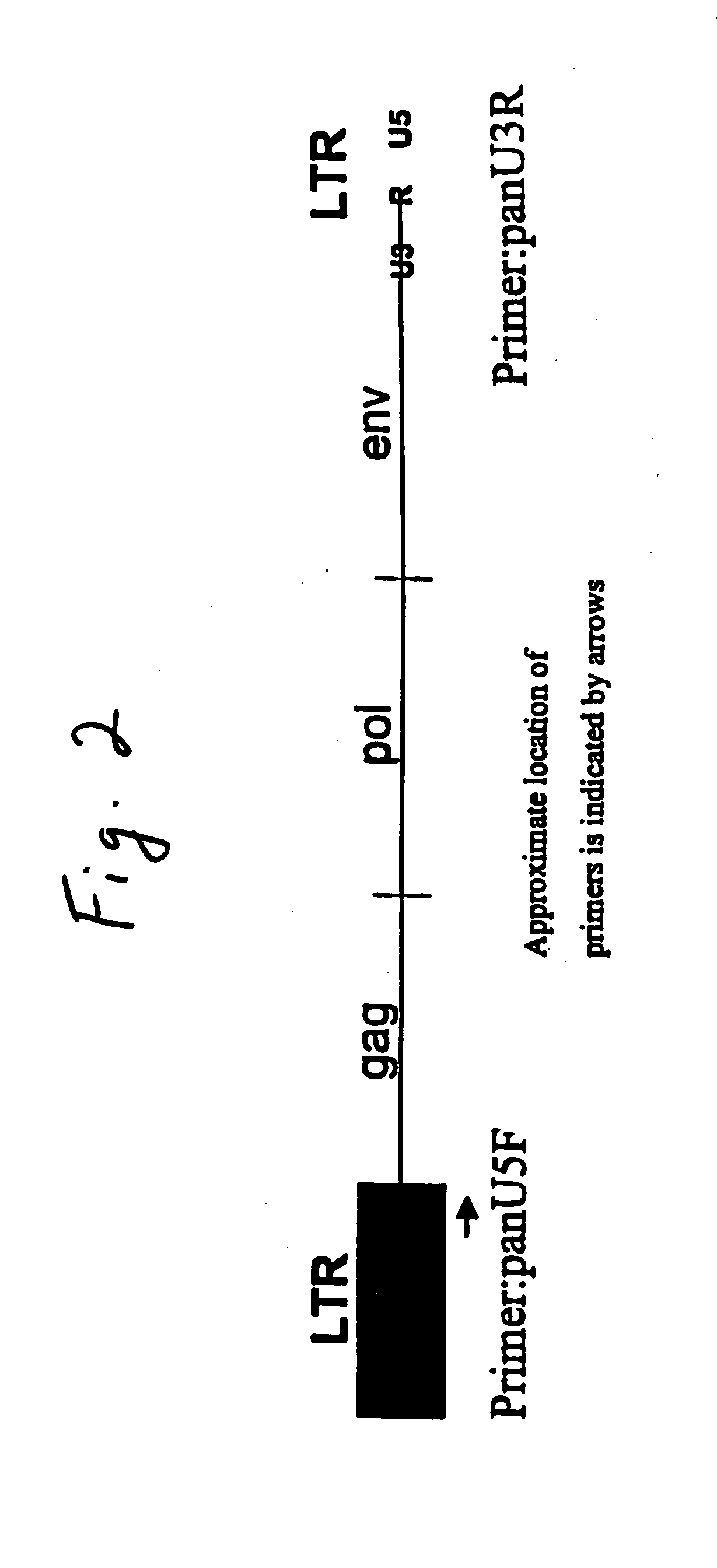
Swine defective for transmission of porcine endogenous retrovirus and uses thereof
a technology of porcine endogenous retrovirus and porcine swine, which is applied in the field of inbred swine, can solve the problems of significant morbidity, artificially restricted waiting list, and shortage of organs available for transplantation worldwide, and achieve the effect of microbiological safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Co-Culture Experiments Showing that D / D Miniature Swine Do Not Produce Human Tropic PERV
[0048] In this assay the following co-culture conditions were performed. It should be noted that this assay varied in some important details from the established methodologies previously described in the literature [13]. Following purification from fresh blood from D / D haplotype miniature swine, 5×106 lymphocytes were stimulated with PHA and PMA (with or without pretreatment by 2 Gy irradiation) and co-cultured directly with either 1×106 human or porcine cells. The porcine kidney cell line PK15 was used as a positive control for the co-culture assays because these cells produce two families of PERV that can replicate in human cells [11,12]. Control cultures of human and porcine target cells alone were also initiated to ensure absence of retroviral contamination and that the PERV detected originated from the swine PBMC and not from the porcine target cells themselves. Shown in Table 1 are the RT ...
example 2
Increased Stringency In Coculture Experiments Confirms that D / D Miniature Swine Do Not Produce Human Tropic PERV
[0050] As a more stringent analysis of PERV transmission to human cells the following stimulation protocol was adopted. PBMCs were isolated from fresh blood from A / A and D / D haplotype animals, resuspended at 106 cells per ml in RPMI medium and stimulated with PHA and PMA. At three days post stimulation, 2×106 uninfected human or porcine indicator cells were added to 107 PBMC. This co-culture procedure was repeated on further aliquots of cells 24 hours later to maximize the period during which PERV may transmit from the PBMC to indicator cells. The two separate co-cultures were then pooled on day 7 and maintained in culture. RT production was assessed by PERT assay.
[0051] Significantly, the D / D haplotype cells from another individual animal again did not produce virus that transmitted to human cells. Under these increased stringency conditions, both A / A as well as D / D hap...
example 3
Identification of Primers for Use In PCR of Near Full-Length PERV Sequences
Primer Design
[0069] In accordance with the present invention, PCR primers with capacity to amplify near full-length PERV sequences were designed for the purpose of amplification of all known C-type PERV nucleotide sequences and were aligned using the program GeneWorks. For alignment the following PERV nucleotide sequences were retrieved using Blast searches in Genbank (http: / / www.ncbi.nlm.nih.gov / BLAST / ).
Virus DesignationAccession Number.PERV-AAF038601PERV-BY17013PERV-CAF038600
[0070] The LTR regions of PERV-A, -B and -C were aligned. In these LTR regions, nucleotide sequences common to all three proviruses were identified for further analysis. Each nucleotide sequence was then run on the Program Genosys Oligo Calculation in order to determine their suitability as a PCR primer (http: / / www.genosys.com). The suitability criteria included: a length between 20 and 28 bp, a Tm of about 65° C., a GC content bet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com