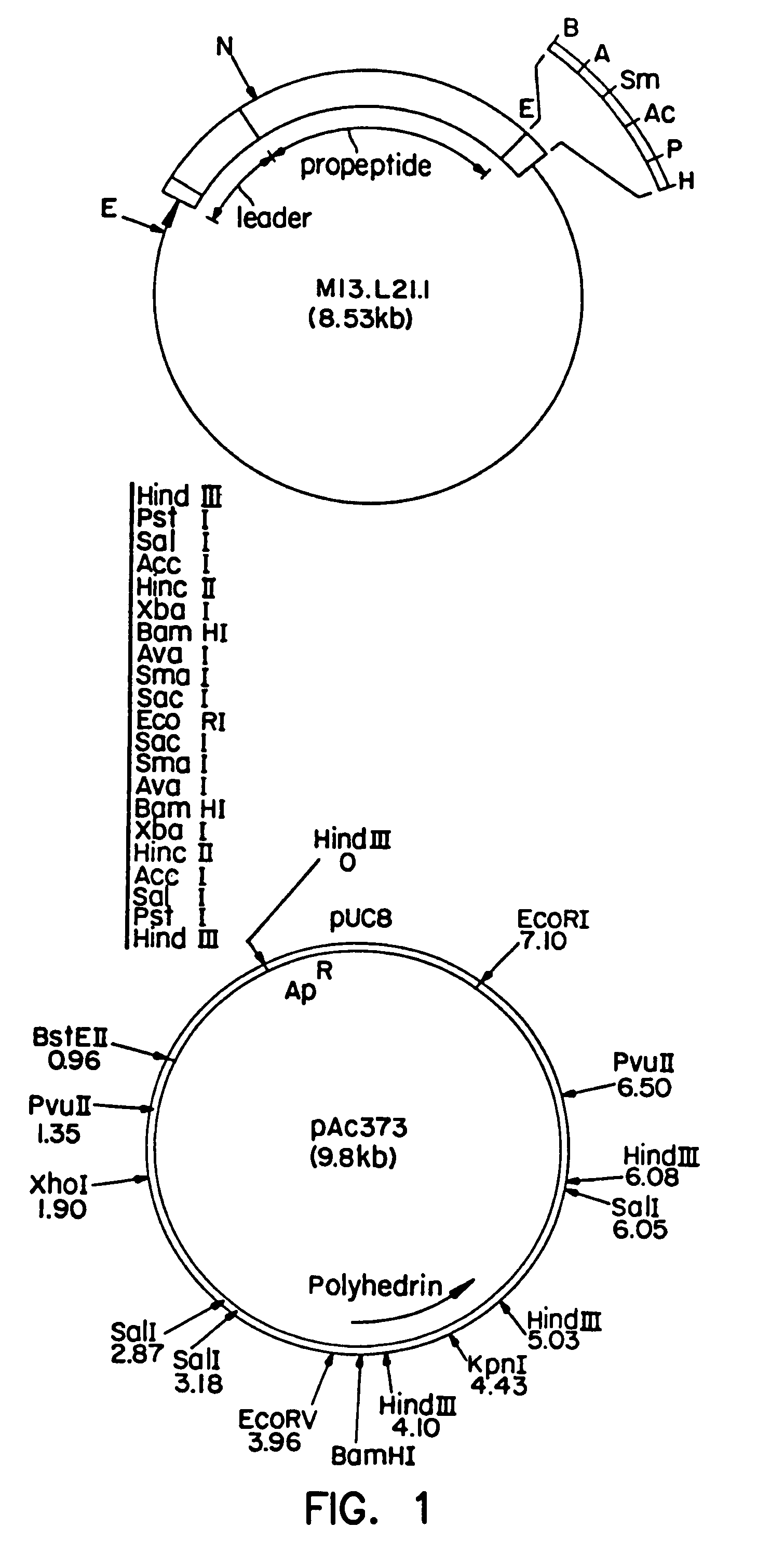
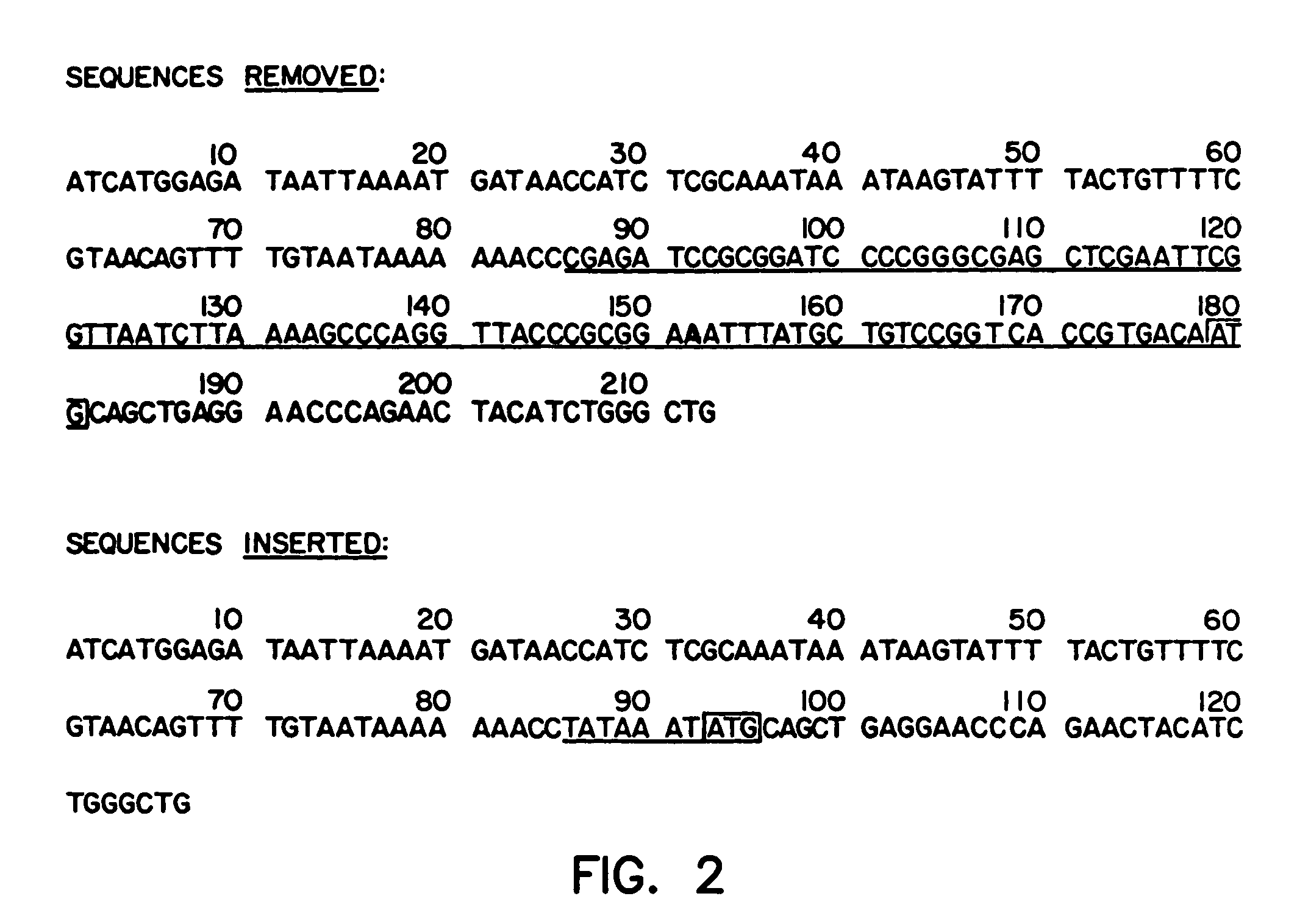
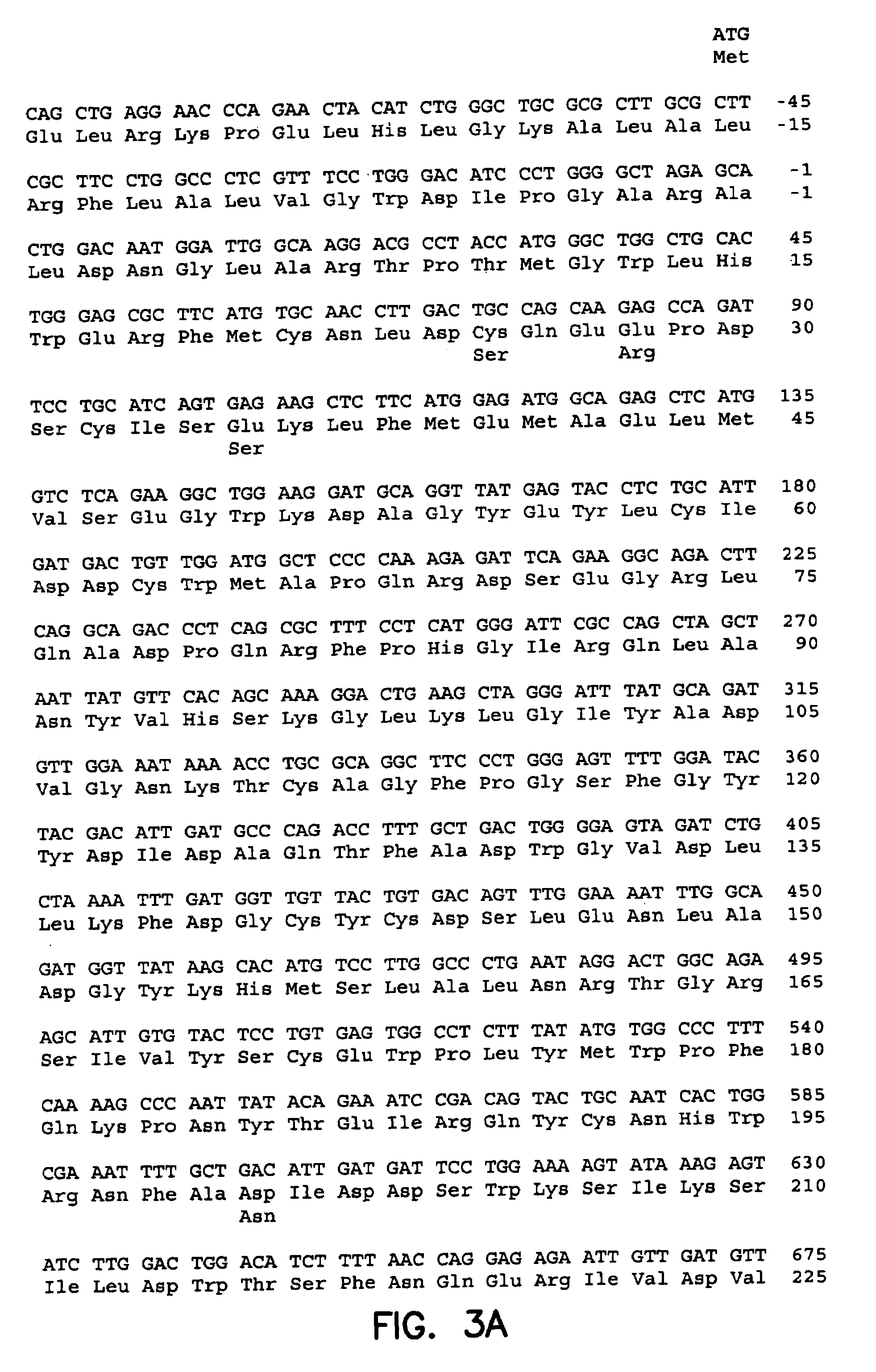
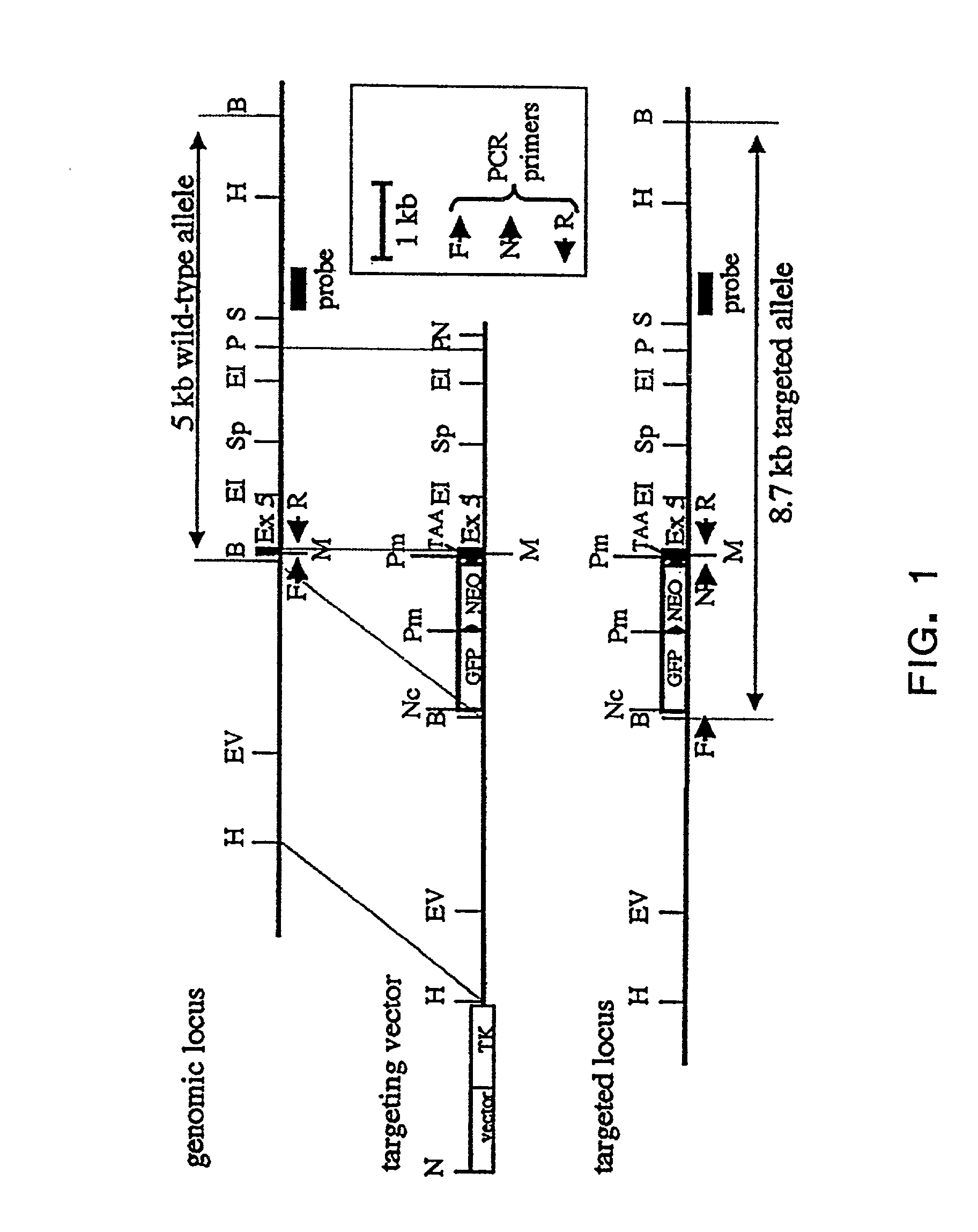
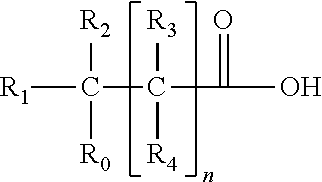Patents
Literature
124 results about "Deficiency of the interleukin-1–receptor antagonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
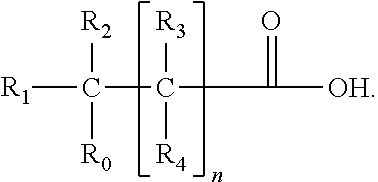
Deficiency of the interleukin-1–receptor antagonist (DIRA) is an autosomal recessive, genetic autoinflammatory syndrome resulting from mutations in IL1RN, the gene encoding the interleukin 1 receptor antagonist. The mutations result in an abnormal protein that is not secreted, exposing the cells to unopposed interleukin 1 activity. This results in sterile multifocal osteomyelitis, periostitis (inflammation of the membrane surrounding the bones), and pustulosis due to skin inflammation from birth.
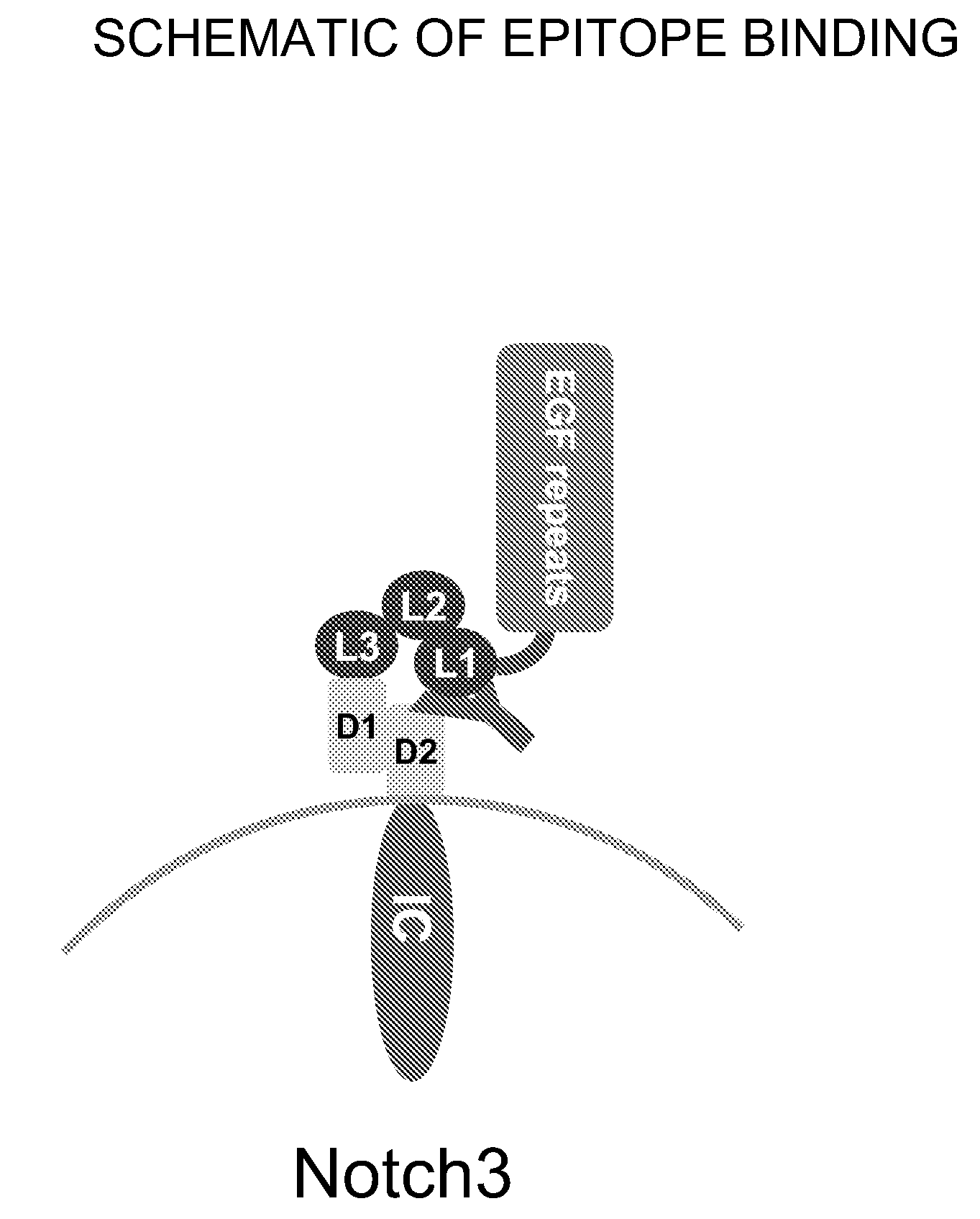
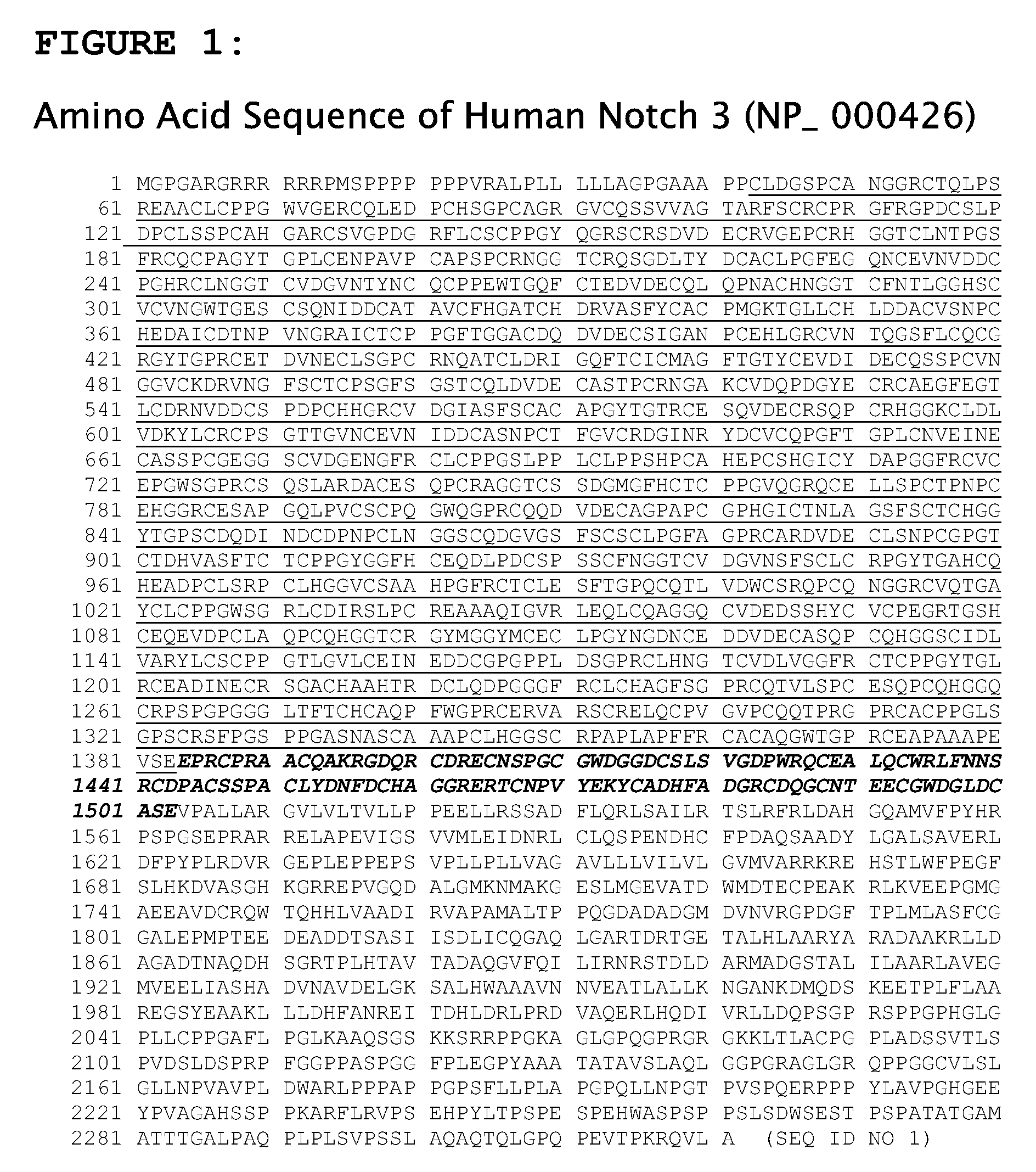
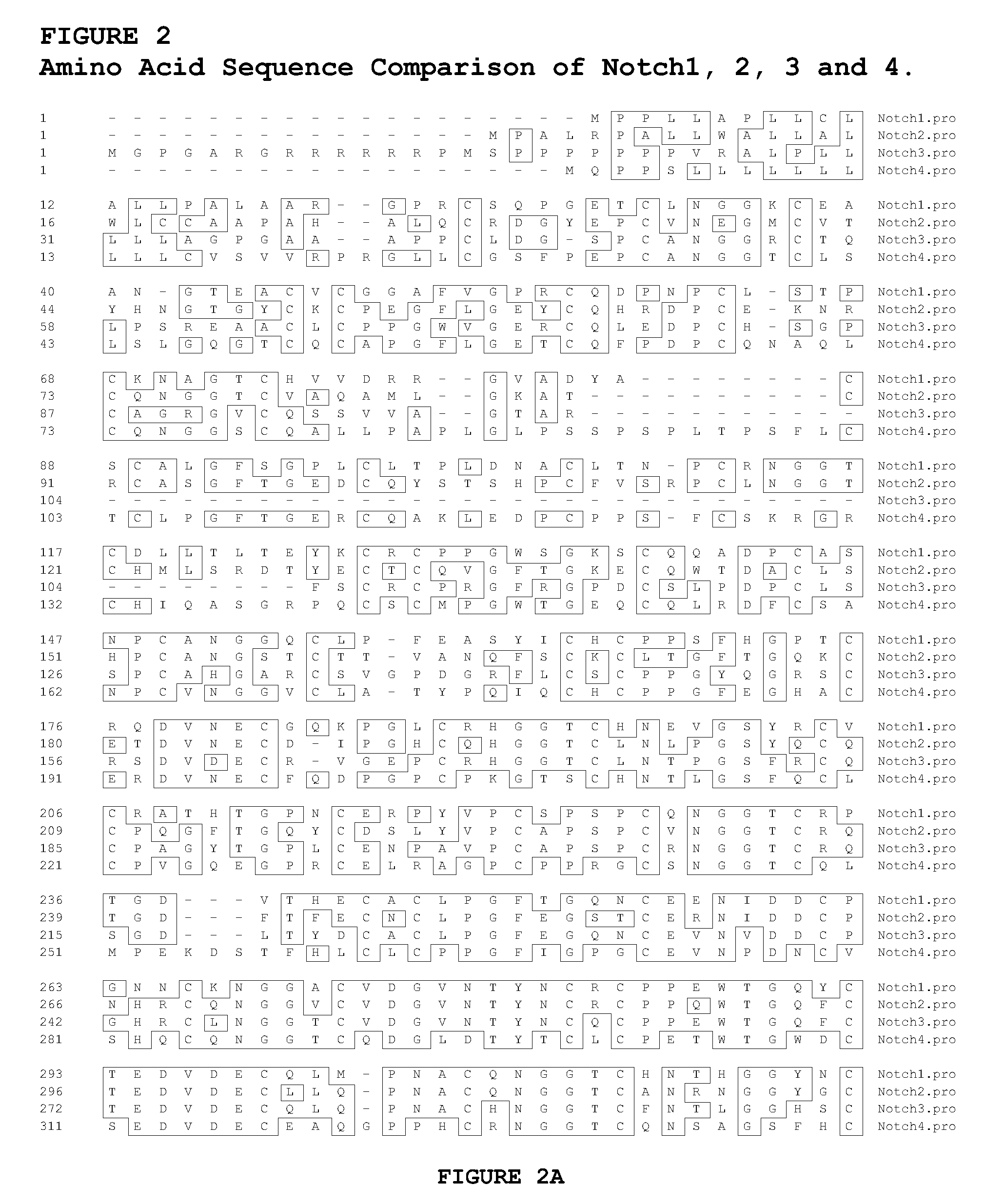
Antagonist Anti-notch3 antibodies and their use in the prevention and treatment of notch3-related diseases
InactiveUS20080226621A1Organic active ingredientsAntipyreticBiological activationConformational epitope
The present invention relates to antagonist antibodies that specifically bind to Notch 3 and inhibit its activation. The present invention includes antibodies binding to a conformational epitope comprising the first Lin12 domain and the second dimerization domain. The present invention also includes uses of these antibodies to treat or prevent Notch 3 related diseases or disorders.
Owner:GENENTECH INC
Methods of gene therapy using herpes viral vectors expressing GM-CSF
A genetically disabled mutant virus has a genome which is defective in respect of a selected gene that is essential for the production of infectious new virus particles, and which carries heterologous genetic material encoding an immunomodulatory protein such as GM-CSF, IL-2, or others, such that the mutant virus can infect normal host cells and cause expression of immunomodulatory protein, but the mutant virus cannot cause production of infectious new virus particles except when the virus infects recombinant complementing host cells expressing a gene that provides the function of the essential viral gene; the site of insertion of the heterologous genetic material encoding the immunomodulatory protein preferably being at the site of the defect in the selected essential viral gene. Uses include prophylactic and therapeutic use in generating an immune response in a subject treated therewith; use in the preparation of an immunogen such as a vaccine for use in tumor therapy; use in the in-vitro expansion of (e.g. virus-specific) cytotoxic T cells; and therapeutic or prophylactic use in corrective gene therapy.
Owner:CANTAB PHARMA RES
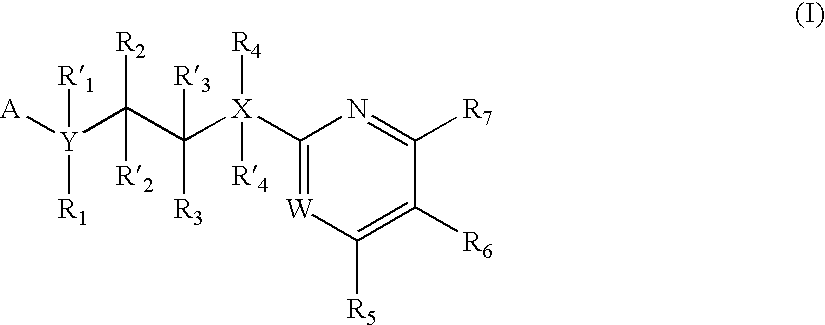
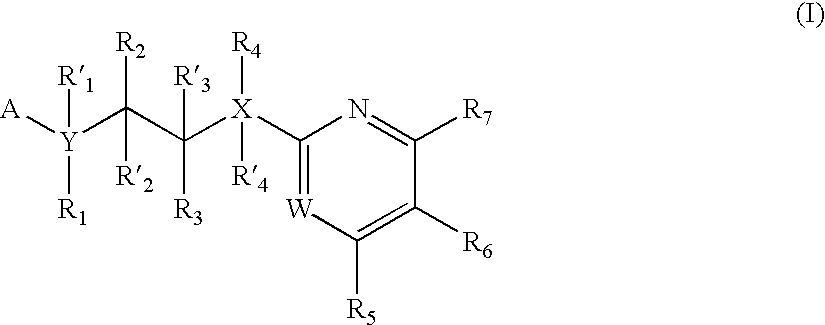
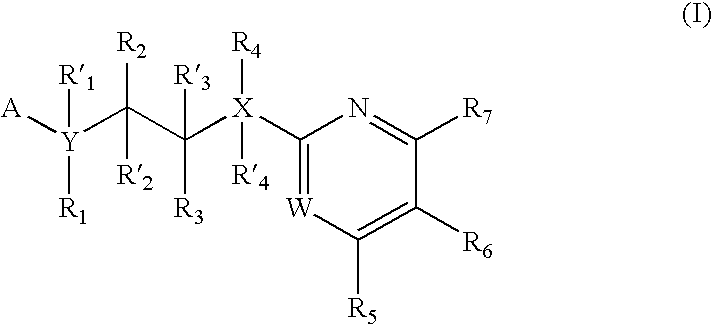
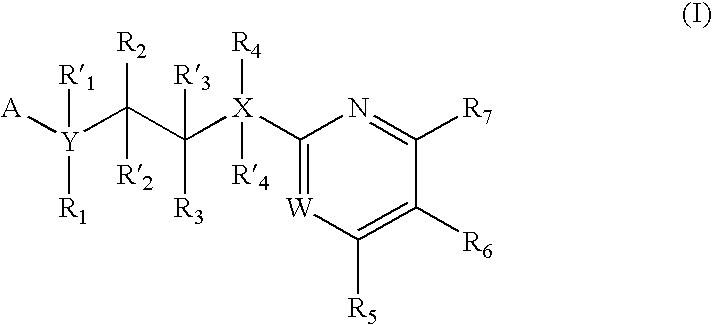
Inhibitors of glycogen synthase kinase 3
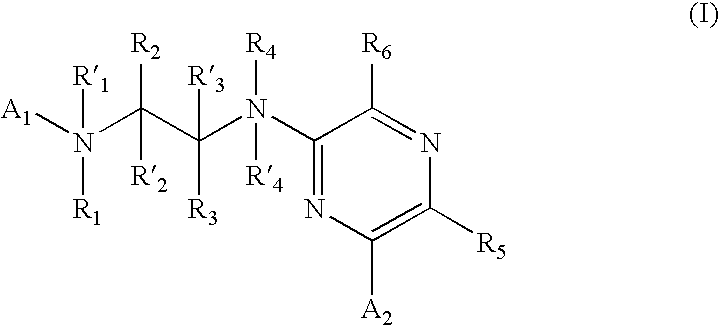
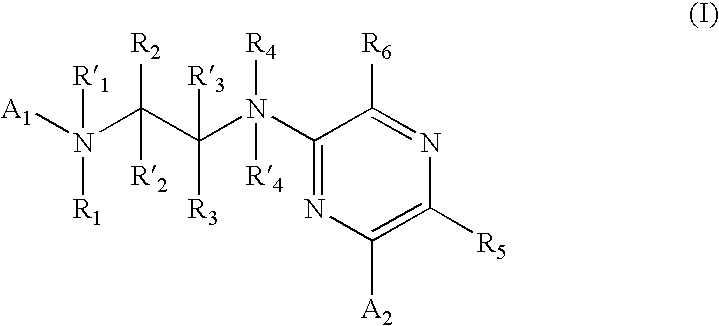
New pyrimidine or pyridine based compounds, compositions and methods of inhibiting the activity of glycogen synthase kinase (GSK3) in vitro and of treatment of GSK3 mediated disorders in vivo are provided. The methods, compounds and compositions of the invention may be employed alone, or in combination with other pharmacologically active agents in the treatment of disorders mediated by GSK3 activity, such as in the treatment of diabetes, Alzheimer's disease and other neurodegenerative disorders, obesity, atherosclerotic cardiovascular disease, essential hypertension, polycystic ovary syndrome, syndrome X, ischemia, traumatic brain injury, bipolar disorder, immunodeficiency or cancer.
Owner:CHIRON CORP
NKX-2.2 and NKX-6.1 transgenic mouse models for diabetes, depression, and obesity
InactiveUS6127598ASuitable for useEasy to detectBiocidePeptide/protein ingredientsInsulin producing cellObesity
The present invention features mouse models for Nkx-2.2 gene function and for Nkx-6.1 gene function, wherein the transgenic mouse is characterized by having a defect in Nkx-2.2 gene function or a defect in Nkx-6.1 gene function (where, because Nkx-2.2 acts upstream of Nkx-6.1, a defect in Nkx-2.2 gene function affects Nkx-6.1 gene function) and by having a decreased number of insulin-producing cells relative to a normal mouse. Where the transgenic mouse contains a defect in Nkx-2.2 gene function, the mouse is further characterized by a decreased number of serotonin-producing cells relative to a normal mouse. The transgenic mice may be either homozygous or heterozygous for the Nkx-2.2 or Nkx-6.1 defect.
Owner:RGT UNIV OF CALIFORNIA
Inhibitors of glycogen synthase kinase 3
Owner:CHIRON CORP
Recombinant alpha-galactosidase A therapy for Fabry disease
InactiveUS7011831B2Peptide/protein ingredientsGenetic material ingredientsBaculovirus expressionInsect cell culture
Fabry disease results from an X-linked deficiency in the enzyme α-galactosidase A. The present invention is directed to recombinant human α-galactosidase A and provides baculovirus expression vectors and recombinant virus that provide stable expression of extracellular and intracellular levels of this enzyme in an insect cell culture. The recombinant-derived enzyme can be used in enzyme replacement therapy to treat Fabry patients. Composition useful in therapeutic administration of α-galactosidase A are also provided.
Owner:SHELBYZYME
Muir-torre-like syndrome in Fhit deficient mice
The invention provides nonhuman transgenic animals with a disrupted FHIT gene. The invention further provides transgenic mice in which one or both Fhit alleles have been inactivated. Preferably, the Fhit-deficient mice develop multiple tumors of both visceral and sebaceous origin, similar to those of Muir-Torre familial cancer syndrome. The present invention further relates to the generation of these transgenic mice and their use as model systems to study the effects of carcinogenic agents in promoting clonal expansion of neoplastic cells in cancers, preferably gastrointestinal cancers of which Muir-Torre syndrome is a subset. The invention further relates to testing therapeutic agents for their efficacy in the prevention and treatment of cancer, preferably gastrointestinal cancer.
Owner:THOMAS JEFFERSON UNIV
Muir-torre-like syndrome in Fhit deficient mice
The invention provides nonhuman transgenic animals with a disrupted FHIT gene. The invention further provides transgenic mice in which one or both Fhit alleles have been inactivated. Preferably, the Fhit-deficient mice develop multiple tumors of both visceral and sebaceous origin, similar to those of Muir-Torre familial cancer syndrome. The present invention further relates to the generation of these transgenic mice and their use as model systems to study the effects of carcinogenic agents in promoting clonal expansion of neoplastic cells in cancers, preferably gastrointestinal cancers of which Muir-Torre syndrome is a subset. The invention further relates to testing therapeutic agents for their efficacy in the prevention and treatment of cancer, preferably gastrointestinal cancer.
Owner:THOMAS JEFFERSON UNIV
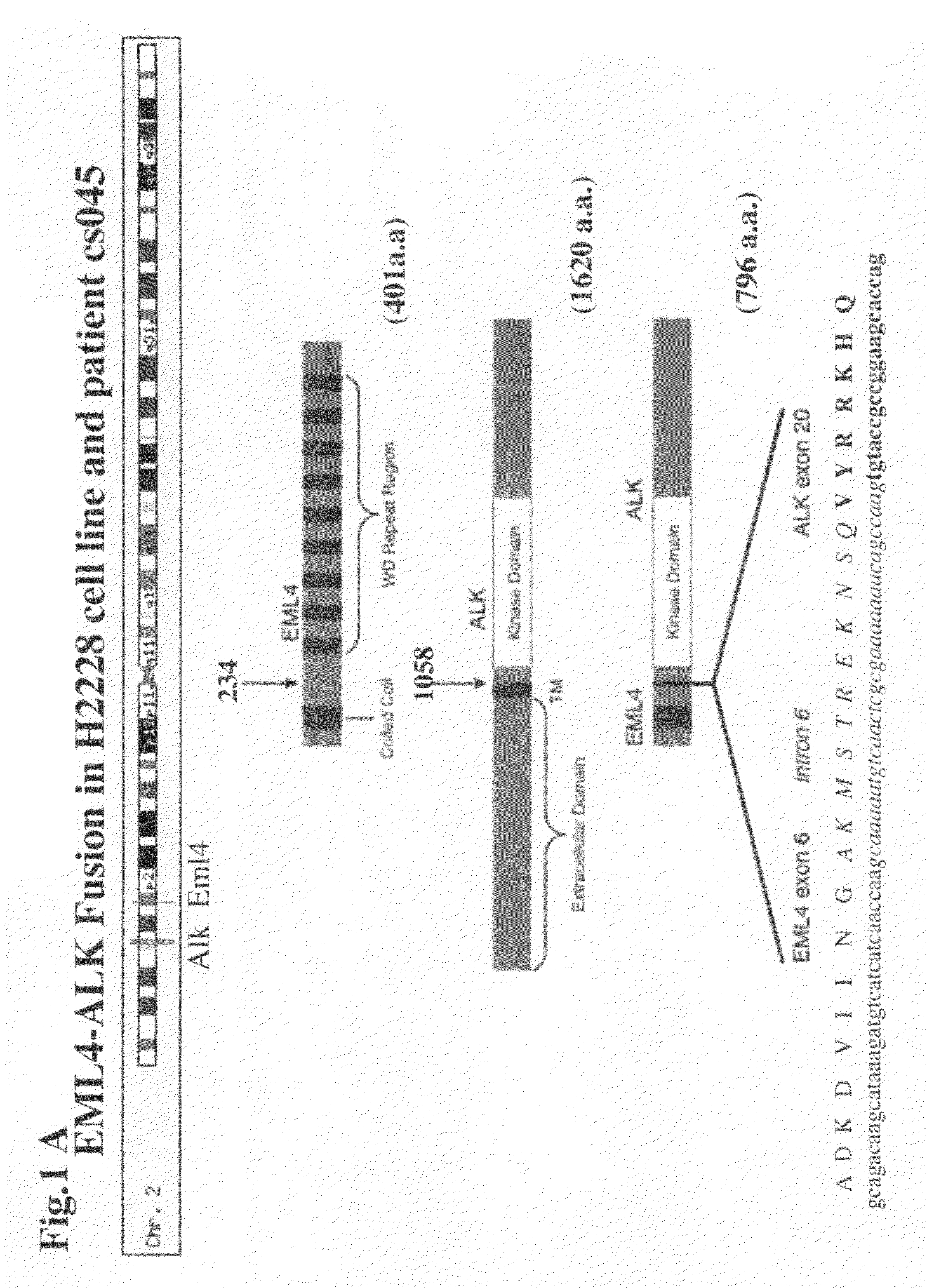
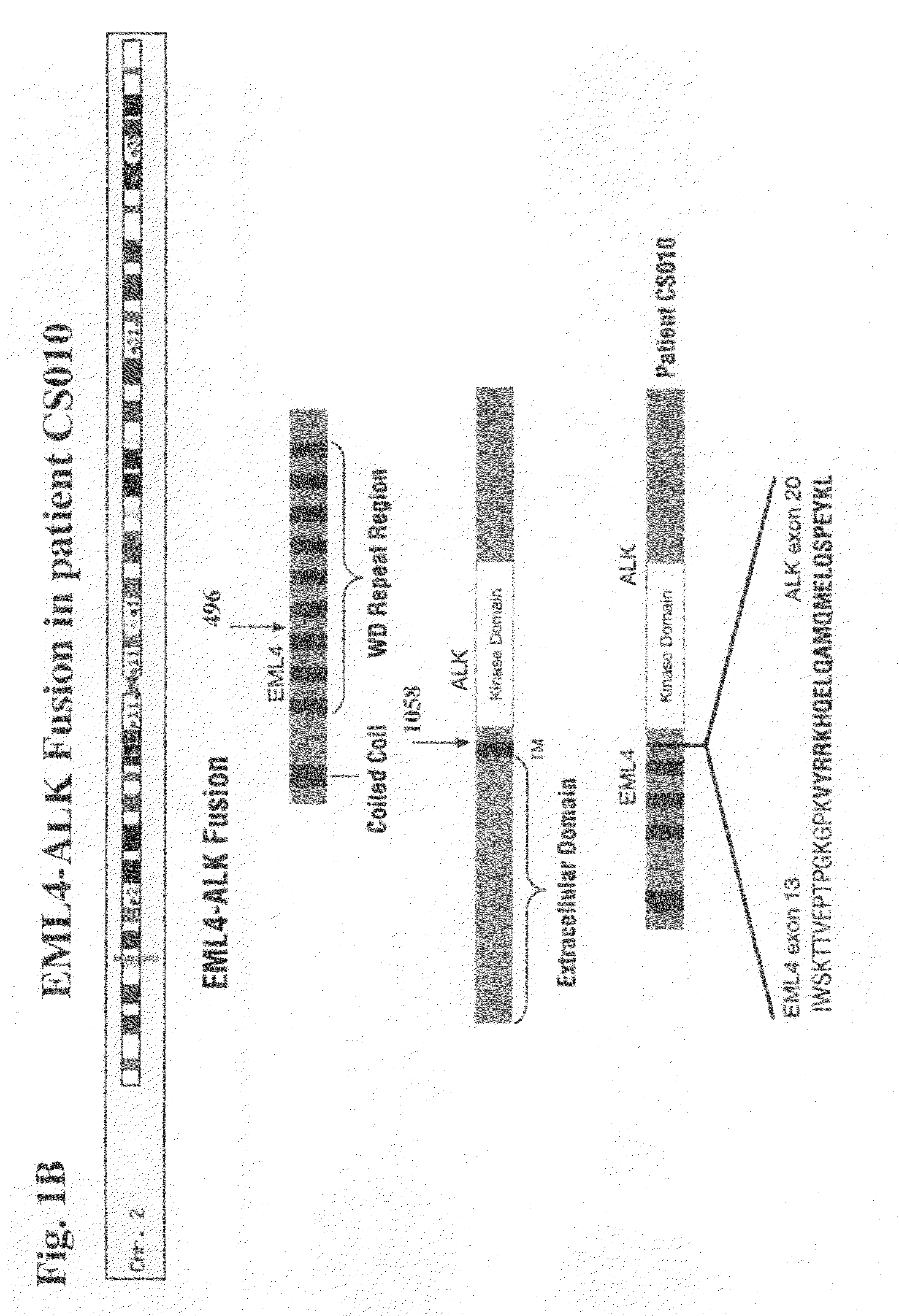
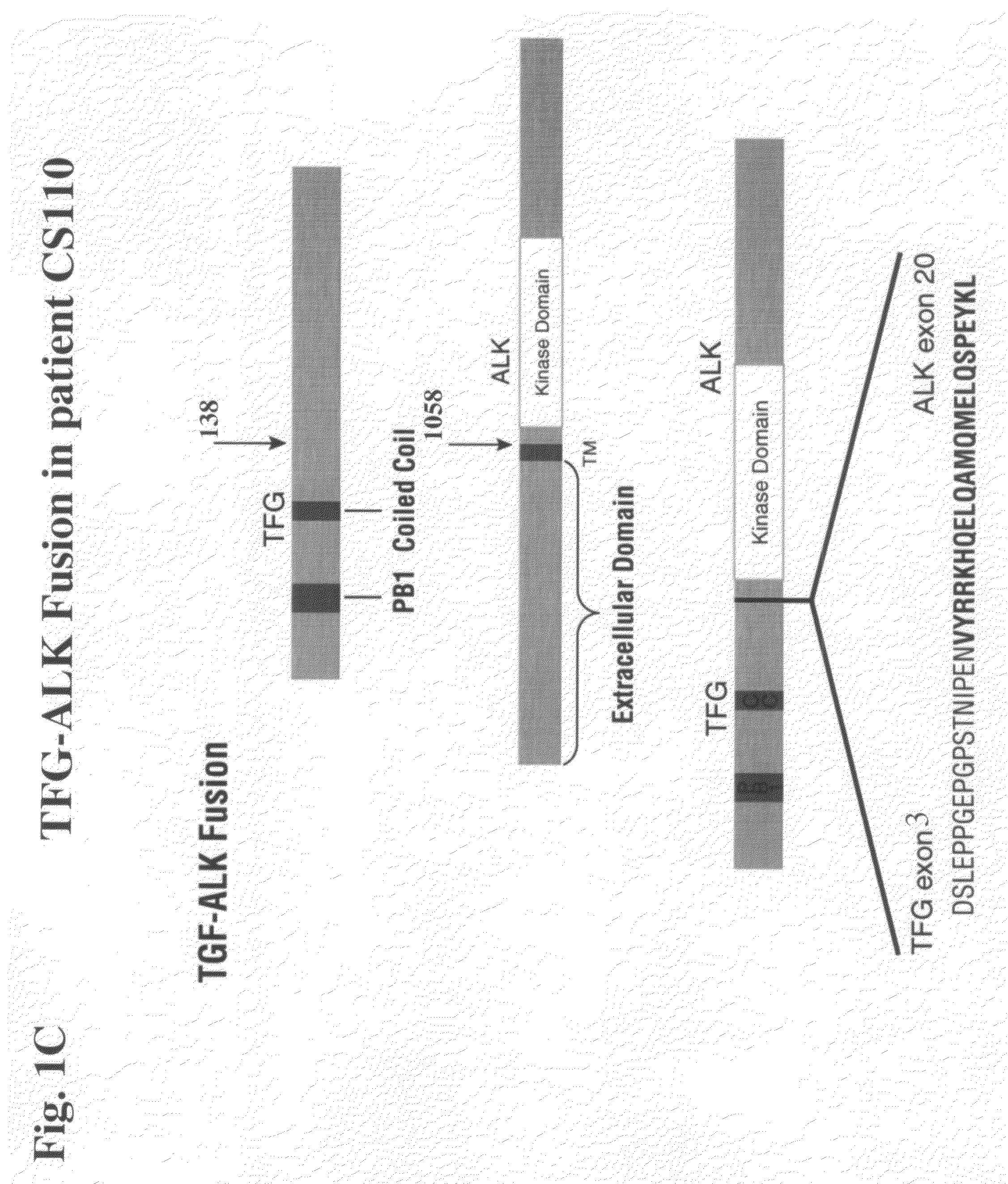
Gene defects and mutant ALK kinase in human solid tumors
In accordance with the invention, novel gene deletions and translocations involving chromosome 2 resulting in fusion proteins combining part of Anaplastic Lymphoma Kinase (ALK) kinase with part of a secondary protein have now been identified in human solid tumors, e.g. non-small cell lung carcinoma (NSCLC). Secondary proteins include Echinoderm Microtubule-Associated Protein-Like 4 (EML-4) and TRK-Fusion Gene (TFG). The EML4-ALK fusion protein, which retains ALK tyrosine kinase activity, was confirmed to drive the proliferation and survival of NSCLC characterized by this mutation. The invention therefore provides, in part, isolated polynucleotides and vectors encoding the disclosed mutant ALK kinase polypeptides, probes for detecting it, isolated mutant polypeptides, recombinant polypeptides, and reagents for detecting the fusion and truncated polypeptides. The disclosed identification of this new fusion protein enables new methods for determining the presence of these mutant ALK kinase polypeptides in a biological sample, methods for screening for compounds that inhibit the proteins, and methods for inhibiting the progression of a cancer characterized by the mutant polynucleotides or polypeptides, which are also provided by the invention.
Owner:CELL SIGNALING TECHNOLOGY
Immuno-reactive peptide CTL epitopes of human cytomegalovirus
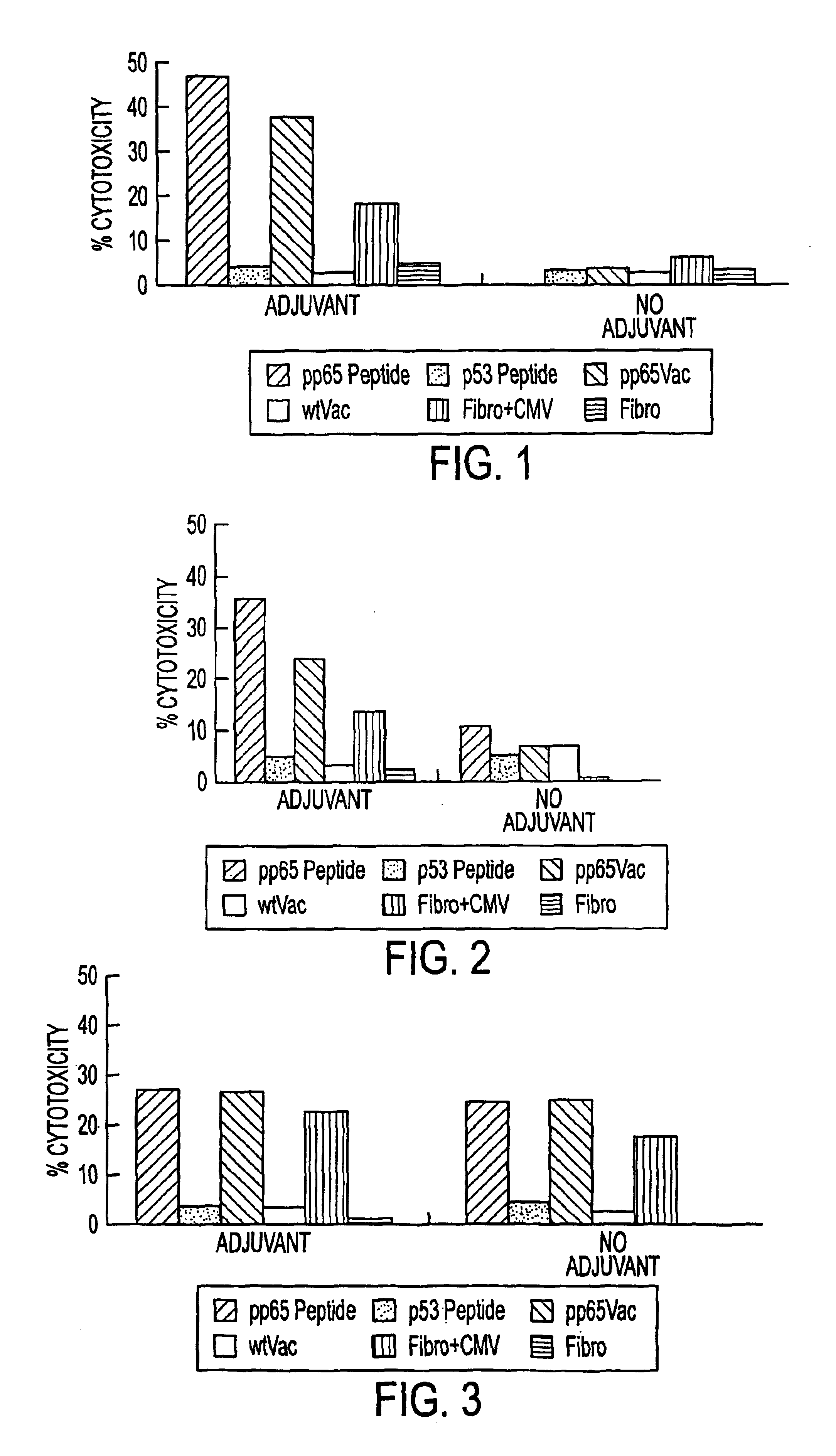
The invention provides a plurality of peptides (and immunologically functional variants thereof) which are immunogenic epitopes recognized by CD8<+> class I MHC restricted cytotoxic T-lymphocytes of patients harboring latent human cytomegalovirus (HCMV) infection. The peptides are capable of activating CTLs and CTLps in the absence of active viral replication, and thus are useful for eliciting a cellular immune response against HCMV by normal and immunodeficient subjects. Peptide and lipopeptide vaccines, with and without adjuvants, also are disclosed. Cellular vaccines comprising the peptides form a further embodiment of this invention.
Owner:CITY OF HOPE
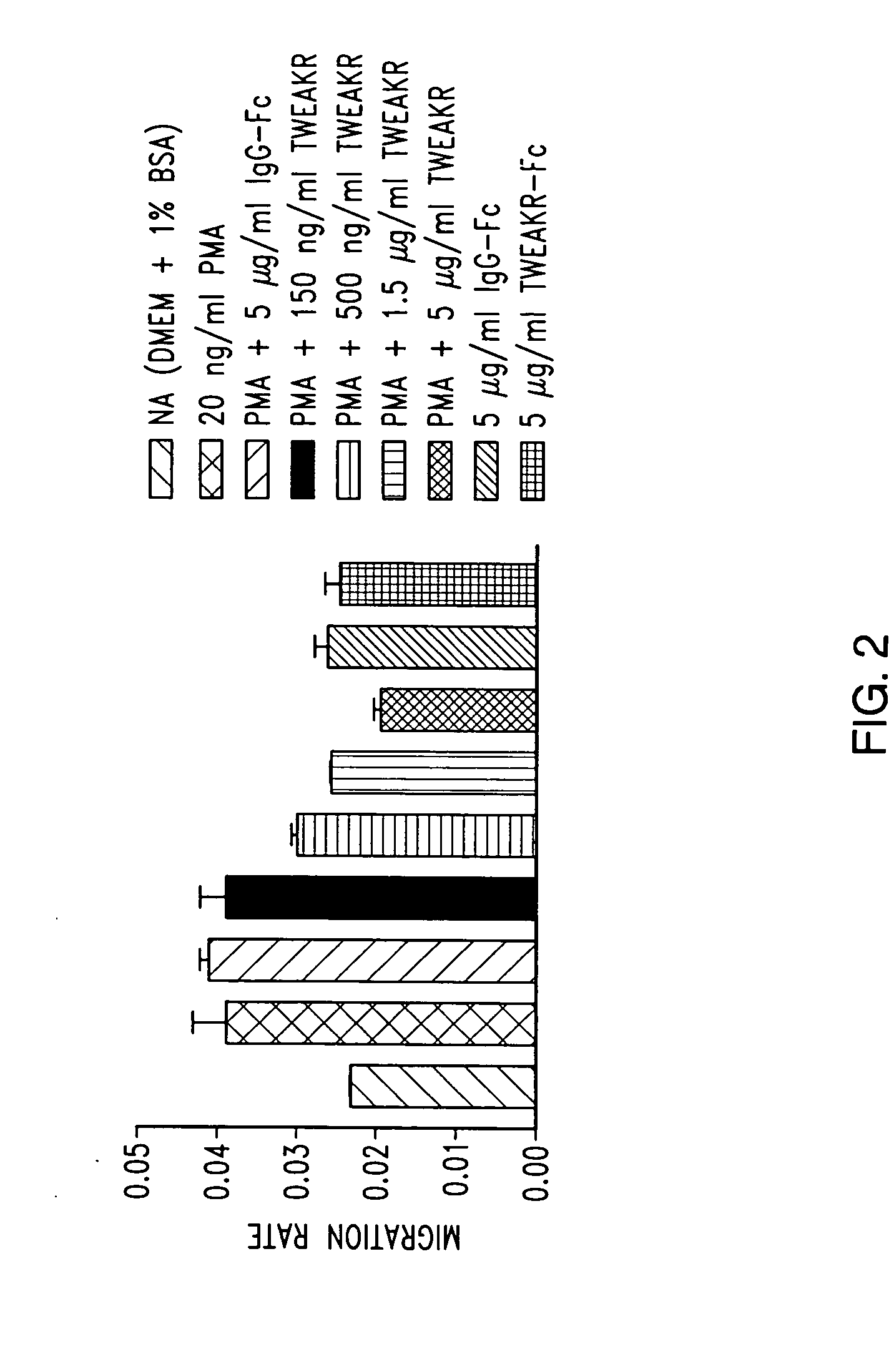
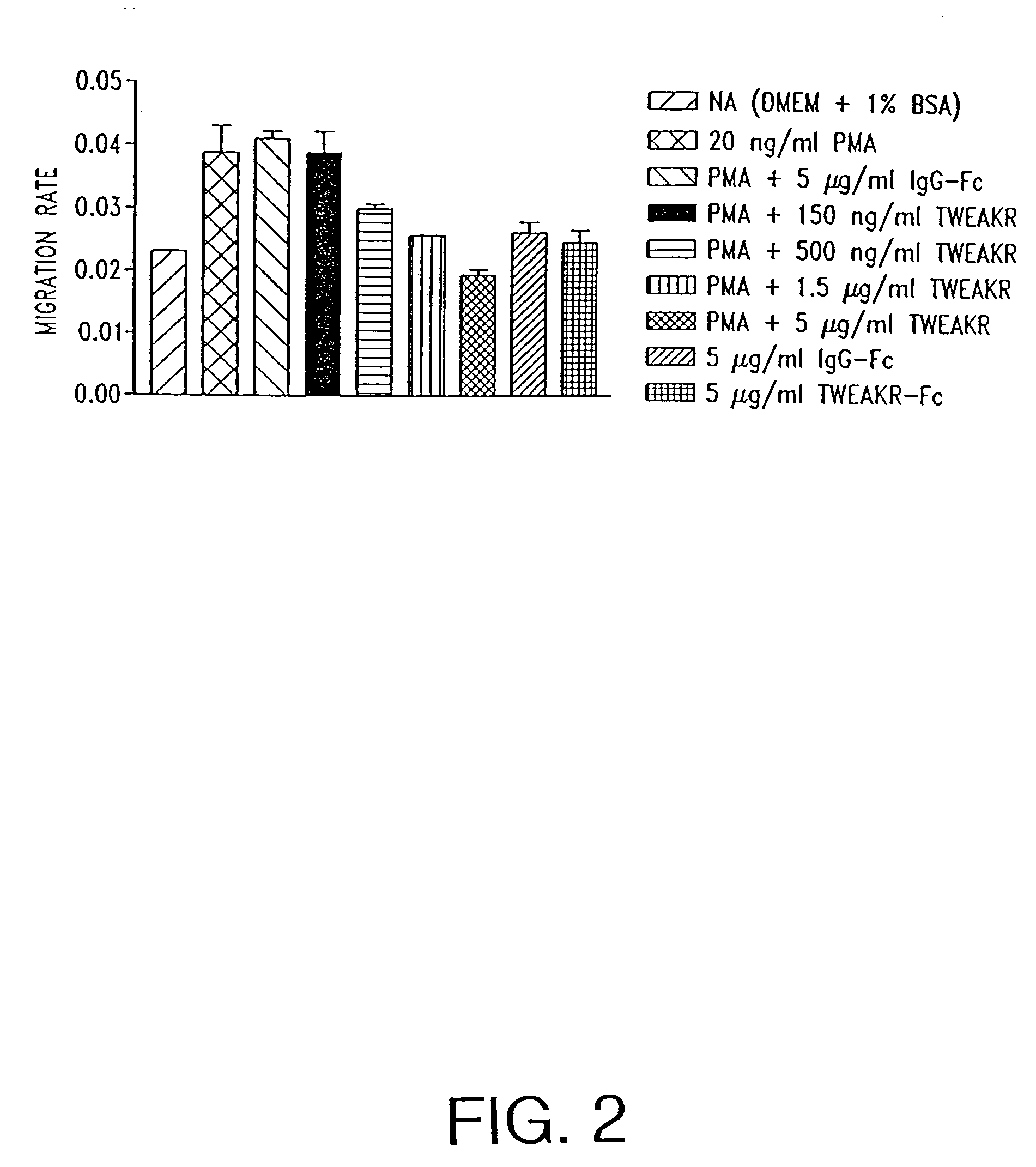
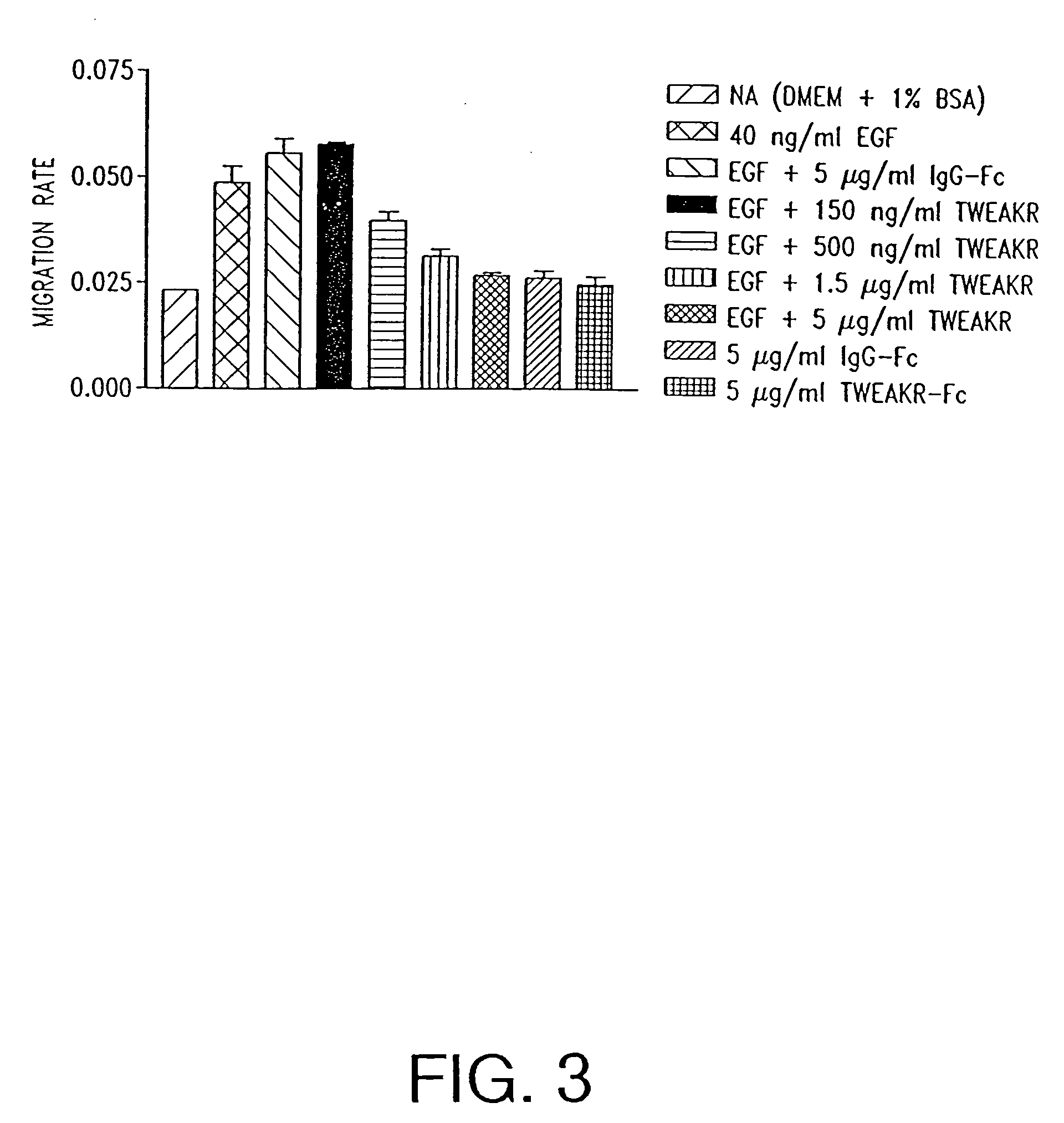
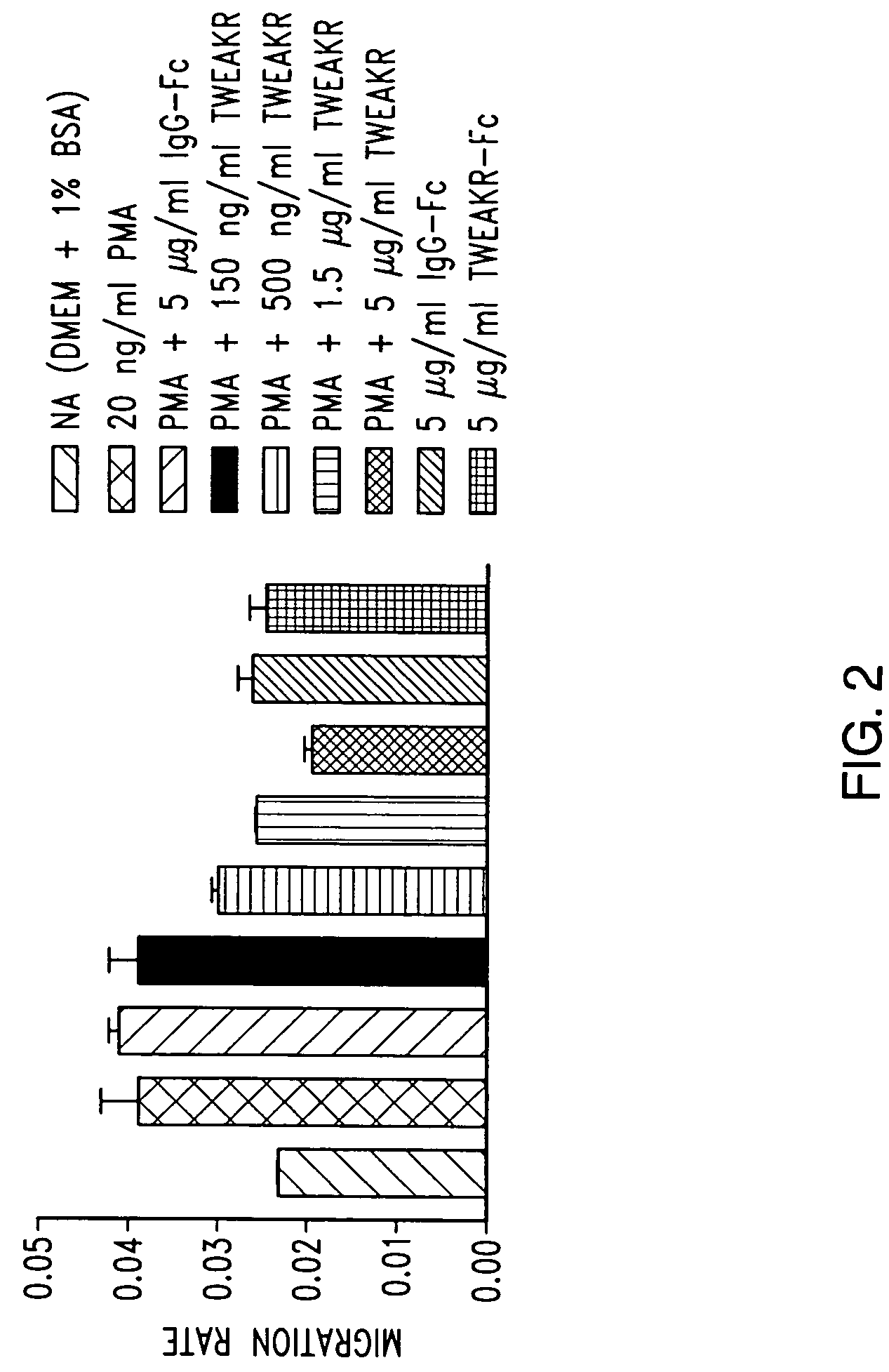
TWEAK receptor
InactiveUS20050208046A1Promote angiogenesisInhibit bindingOrganic active ingredientsFungiAngiogenesis growth factorAgonist
The present invention provides the TWEAK receptor and methods for identifying and using agonists and antagonists of the TWEAK receptor. In particular, the invention provides methods of screening for agonists and antagonists and for treating diseases or conditions mediated by angiogenesis, such as solid tumors and vascular deficiencies of cardiac or peripheral tissue.
Owner:IMMUNEX CORP
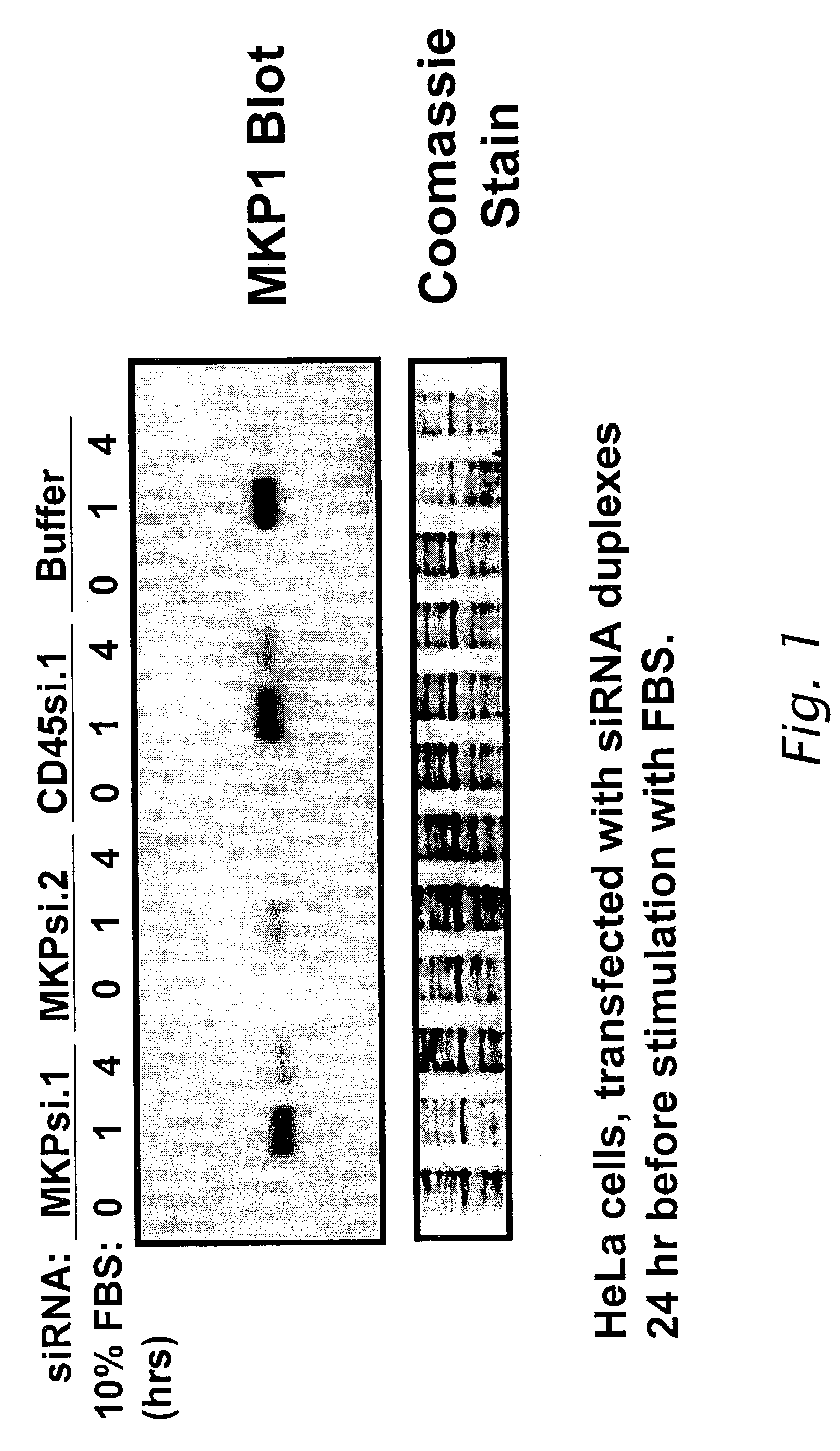
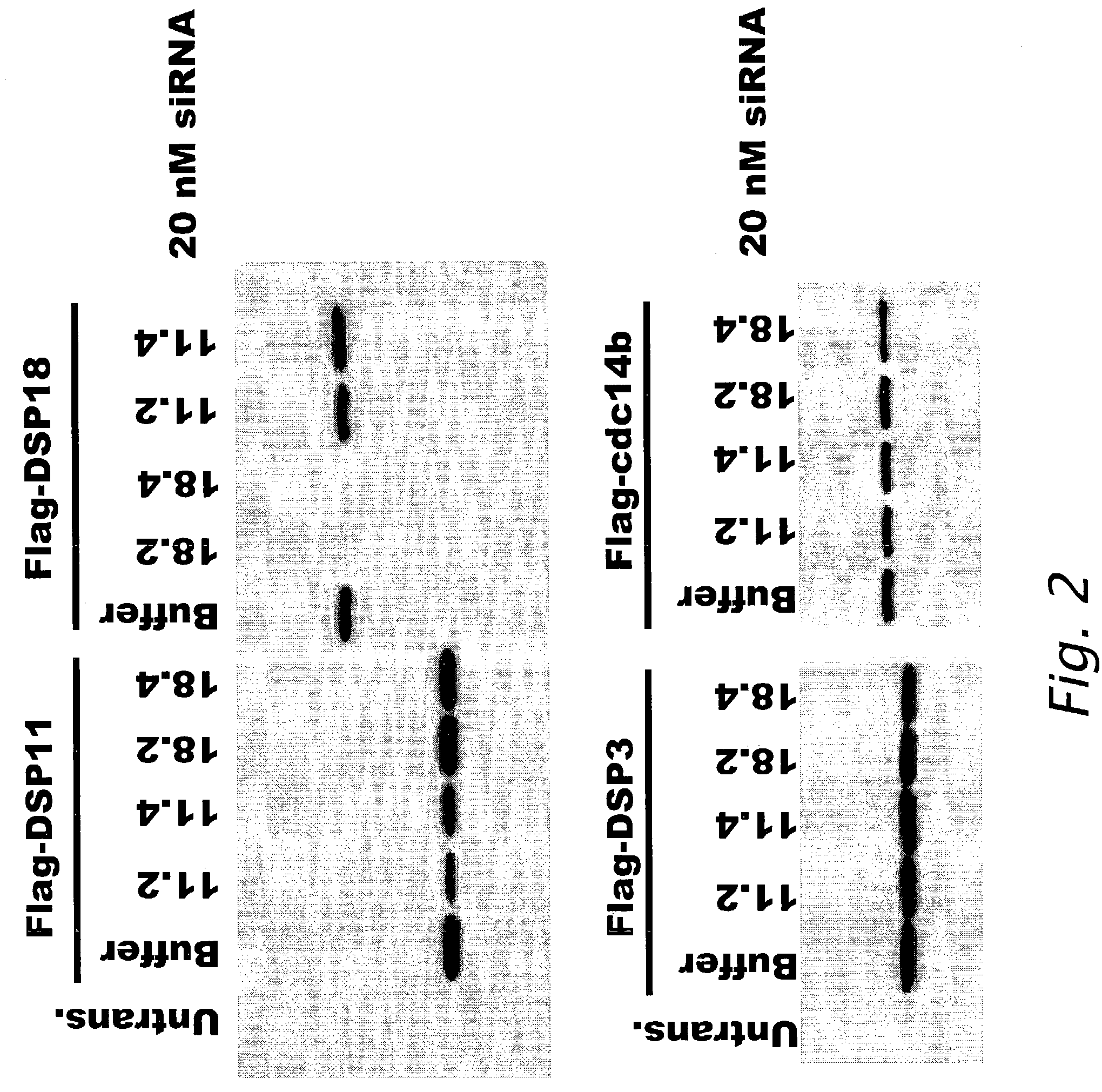
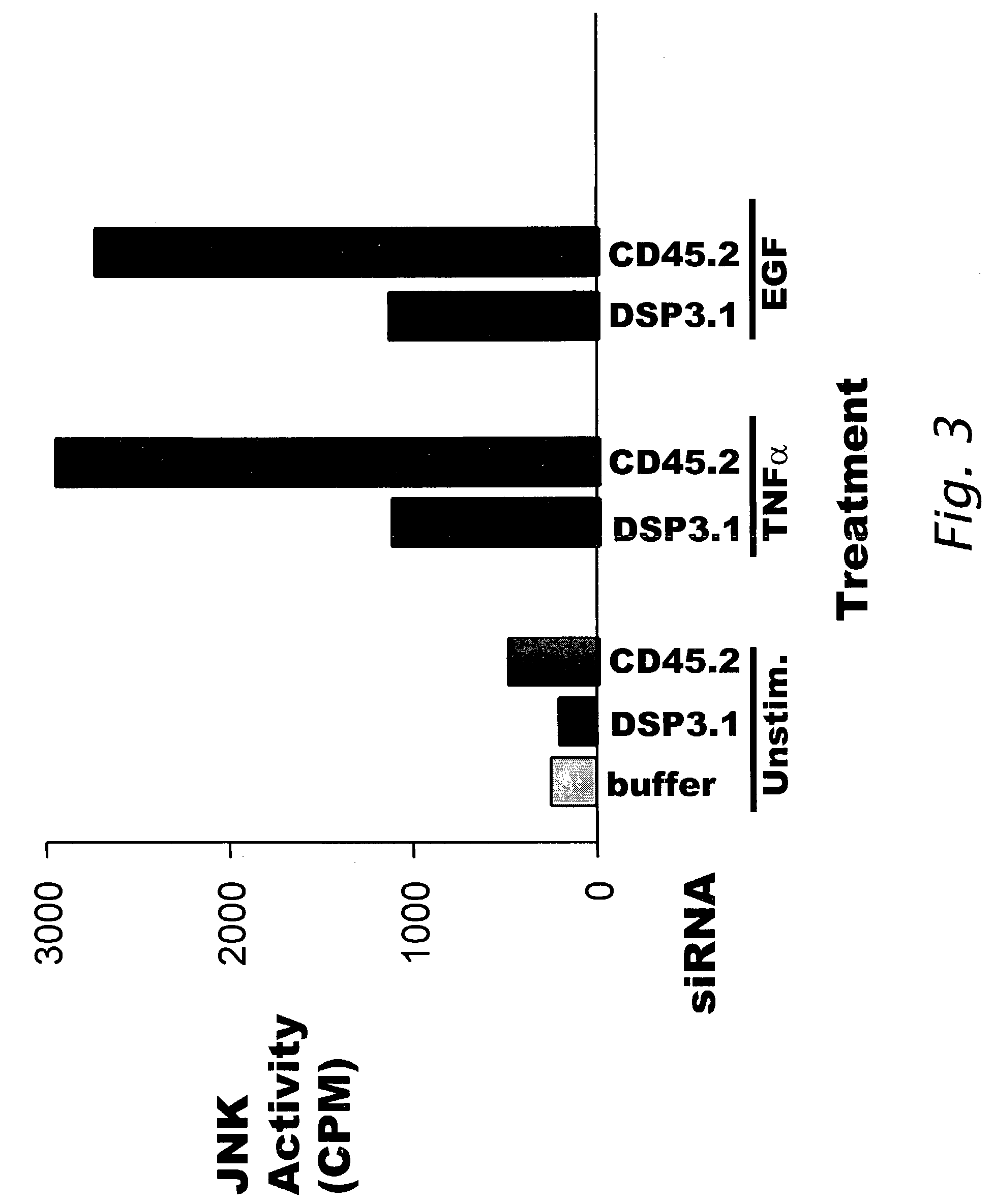
Modulation of biological signal transduction by RNA interference
Compositions and methods relating to small interfering RNA (siRNA) polynucleotides are provided as pertains to modulation of biological signal transduction. Shown are siRNA polynucleotides that interfere with expression of members of the protein tyrosine phosphatase (PTP) class of enzymes that mediate signal transduction, and with certain MAP kinase kinases (MKK). In certain preferred embodiments siRNA modulate signal transduction pathways comprising SHP2, cdc14a / b, cdc25A / B / C, KAP, PTP-ε, PRL-3, CD45, dual specificity phosphatase-3 (DSP-3), MKK-4, and / or MKK-7. Modulation of PTP-mediated biological signal transduction has uses in diseases associated with defects in cell proliferation, cell differentiation and / or cell survival, such as metabolic disorders (including diabetes and obesity), cancer, autoimmune disease, infectious and inflammatory disorders and other conditions. The invention also provides siRNA polynucleotides that interfere with expression of chemotherapeutic target polypeptides, such as DHFR, thymidylate synthetase, and topoisomerase I.
Owner:CEPTYR
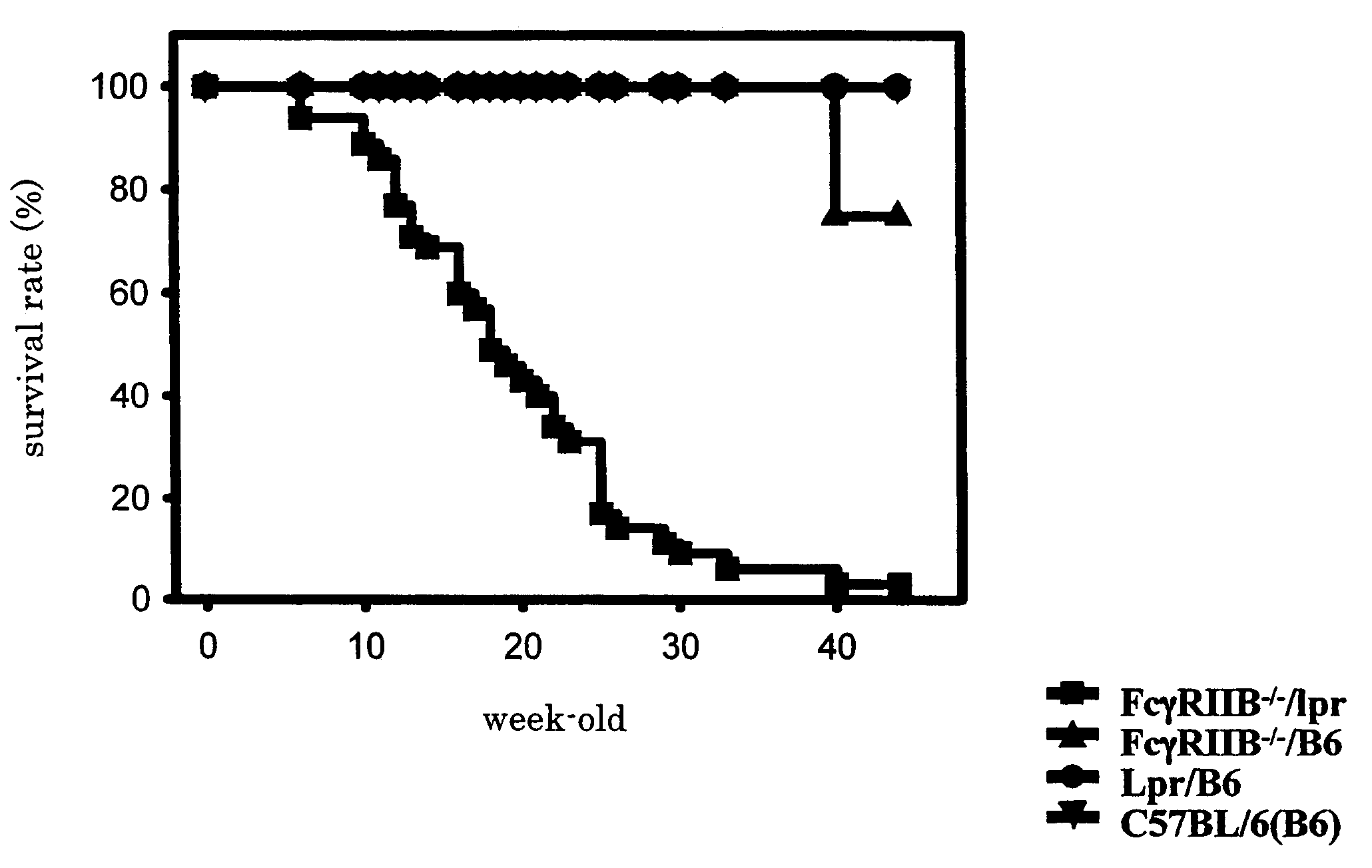
Non-human animal model of systemic lupus erythematosus
InactiveUS7265261B2Microbiological testing/measurementImmunoglobulins against animals/humansScreening methodAnti-dsDNA antibodies
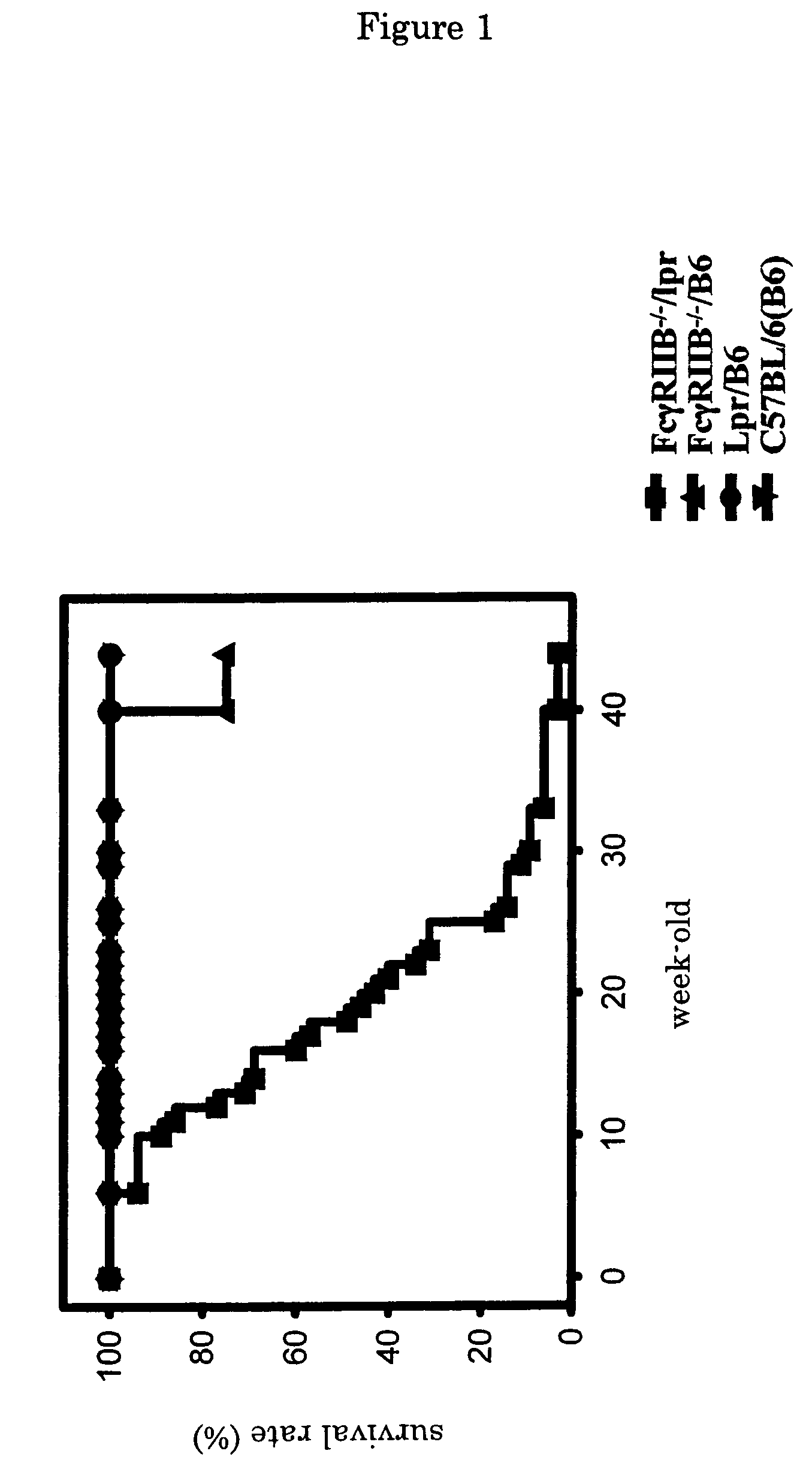
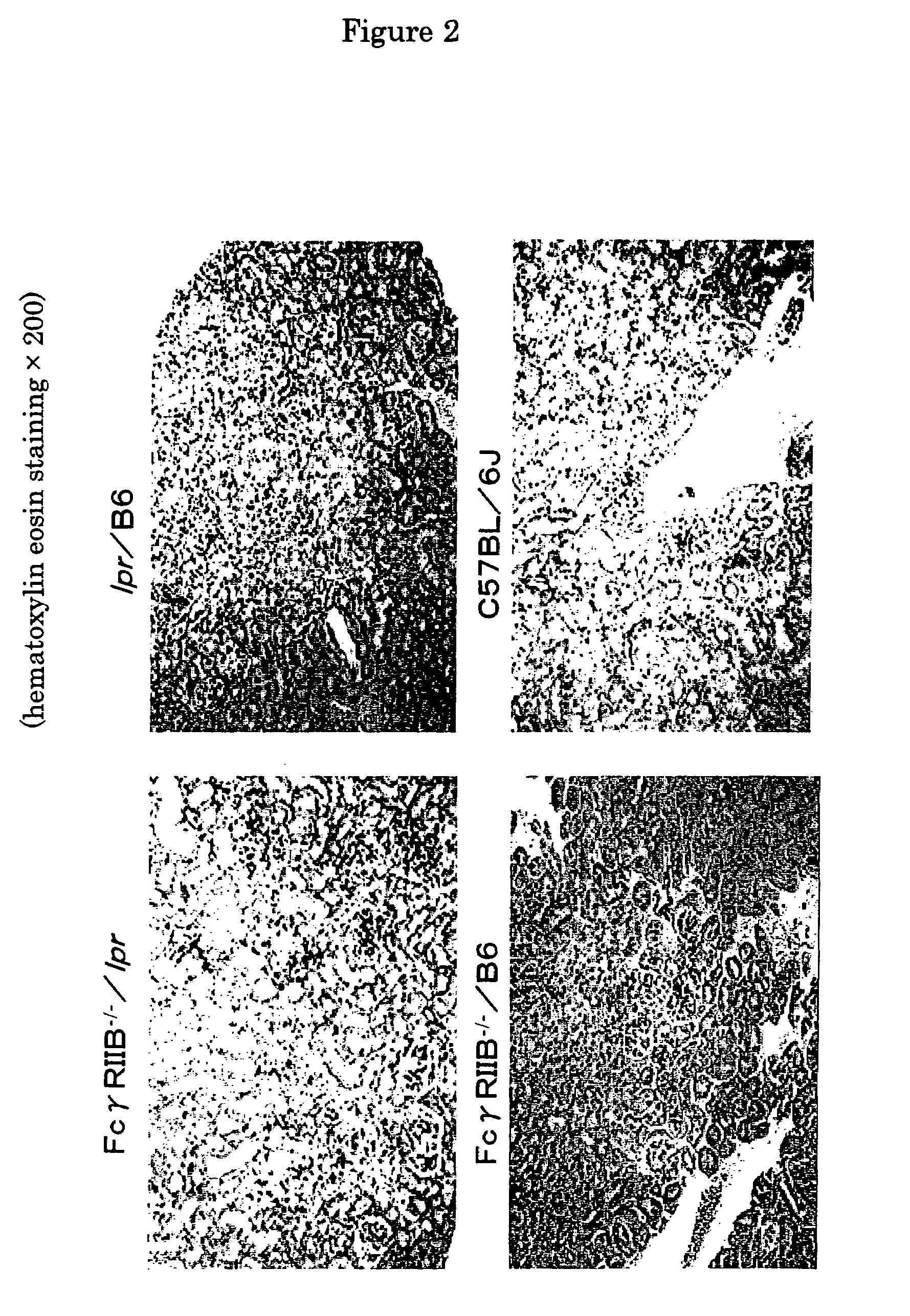
The present invention is to provide a non-human animal model of systemic lupus erythematosus wherein generation of anti-double stranded DNA antibody and anti-single stranded antibody is induced, and that is made to spontaneously develop glomerulonephritis and arthritis, and a screening method for a therapeutic agent for systemic lupus erythematosus wherein the non-human animal model is used. FcγRIIB deficient mouse that is not made to spontaneously develop autoimmune pathology although its autoantibody response is enhanced is backcrossed into C57BL / 6J (B6) mouse for 12 generations to generate FcγRIIB deficient B6 mouse, the FcγRIIB deficient B6 male mouse is intercrossed with lpr / B6 female mouse, and thus obtained FcγRIIB+ / − / lpr+ / − mice were further crossed to generate a mouse model of systemic lupus erythematosus.
Owner:JAPAN SCI & TECH CORP
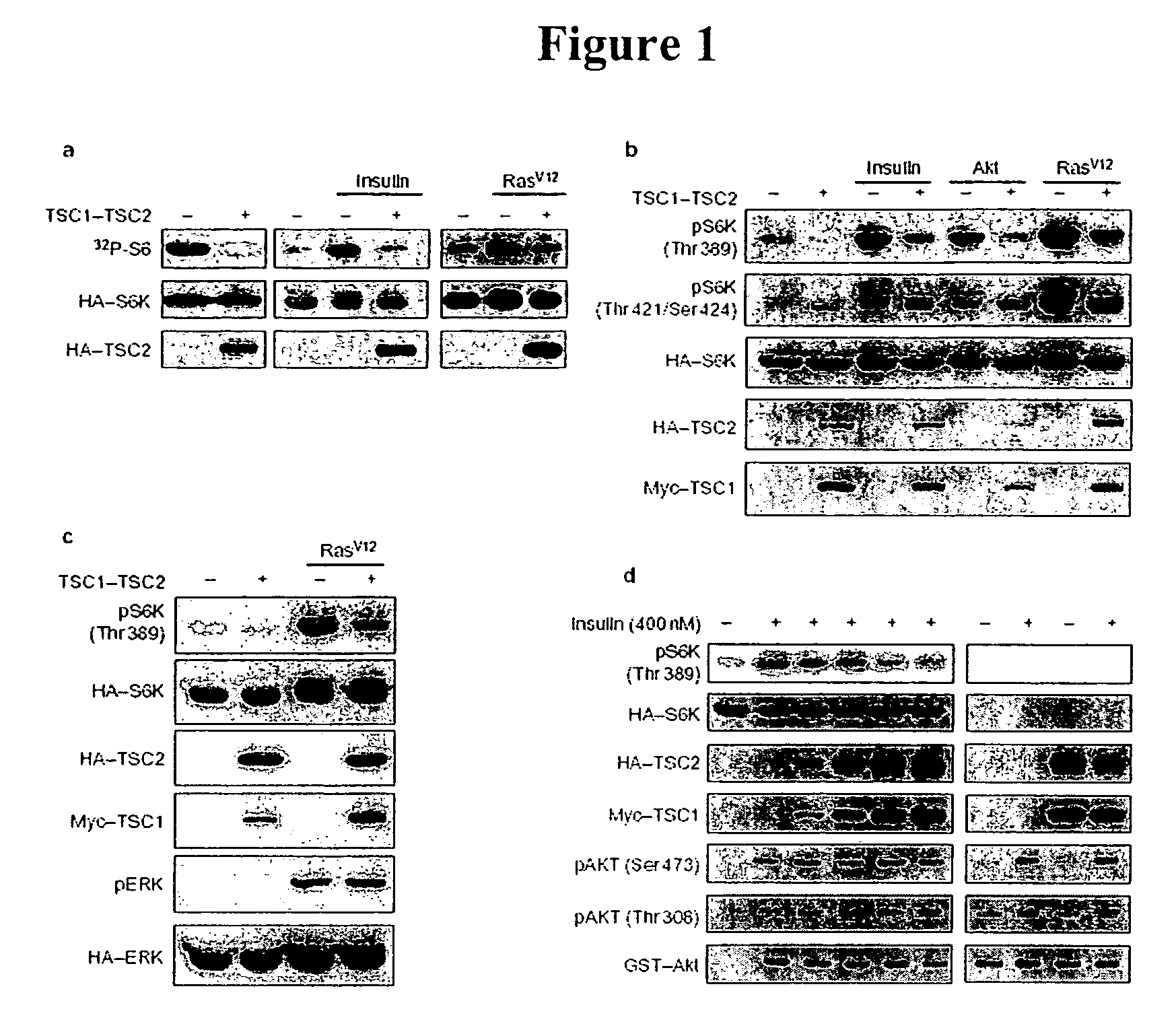
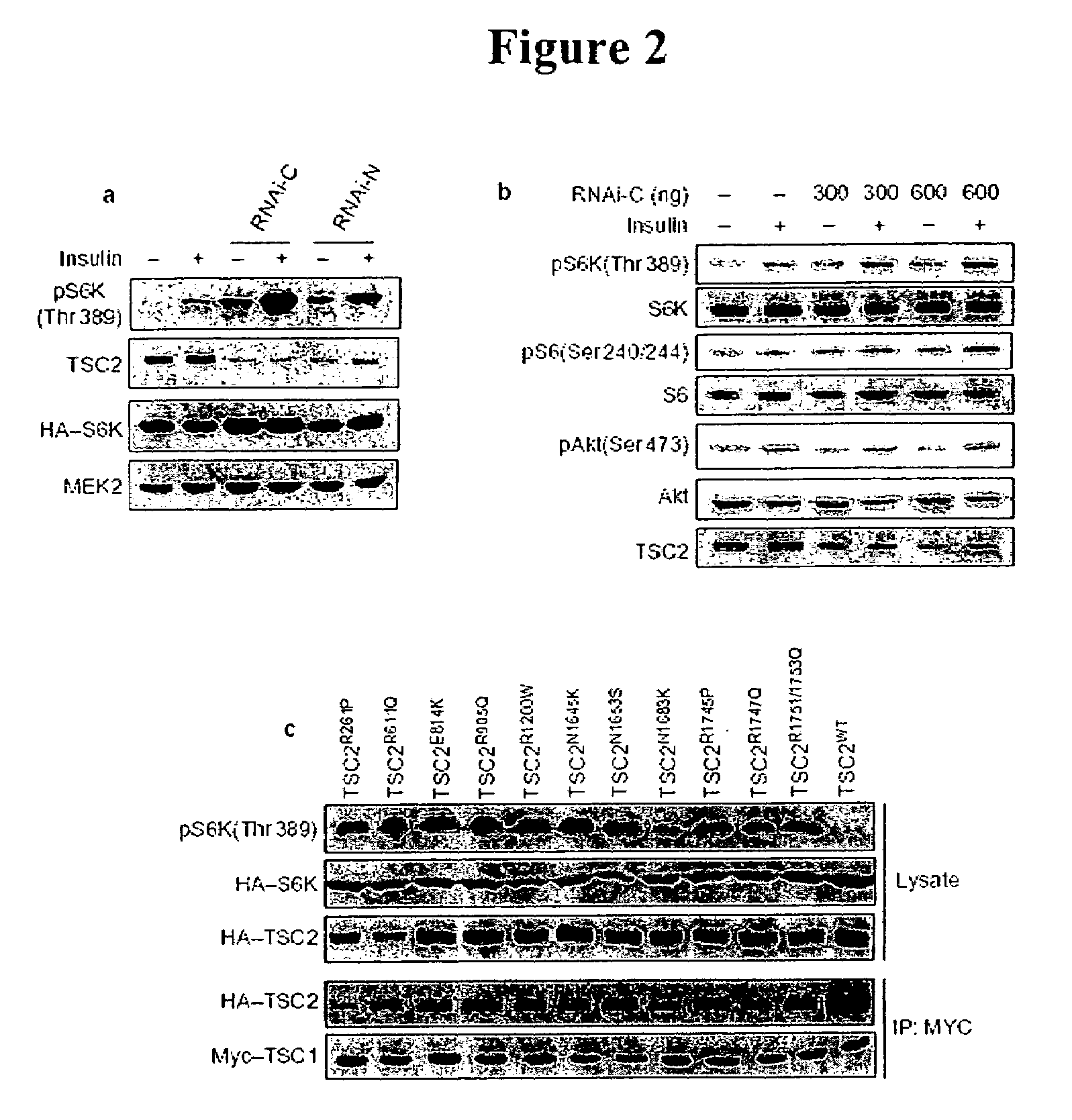
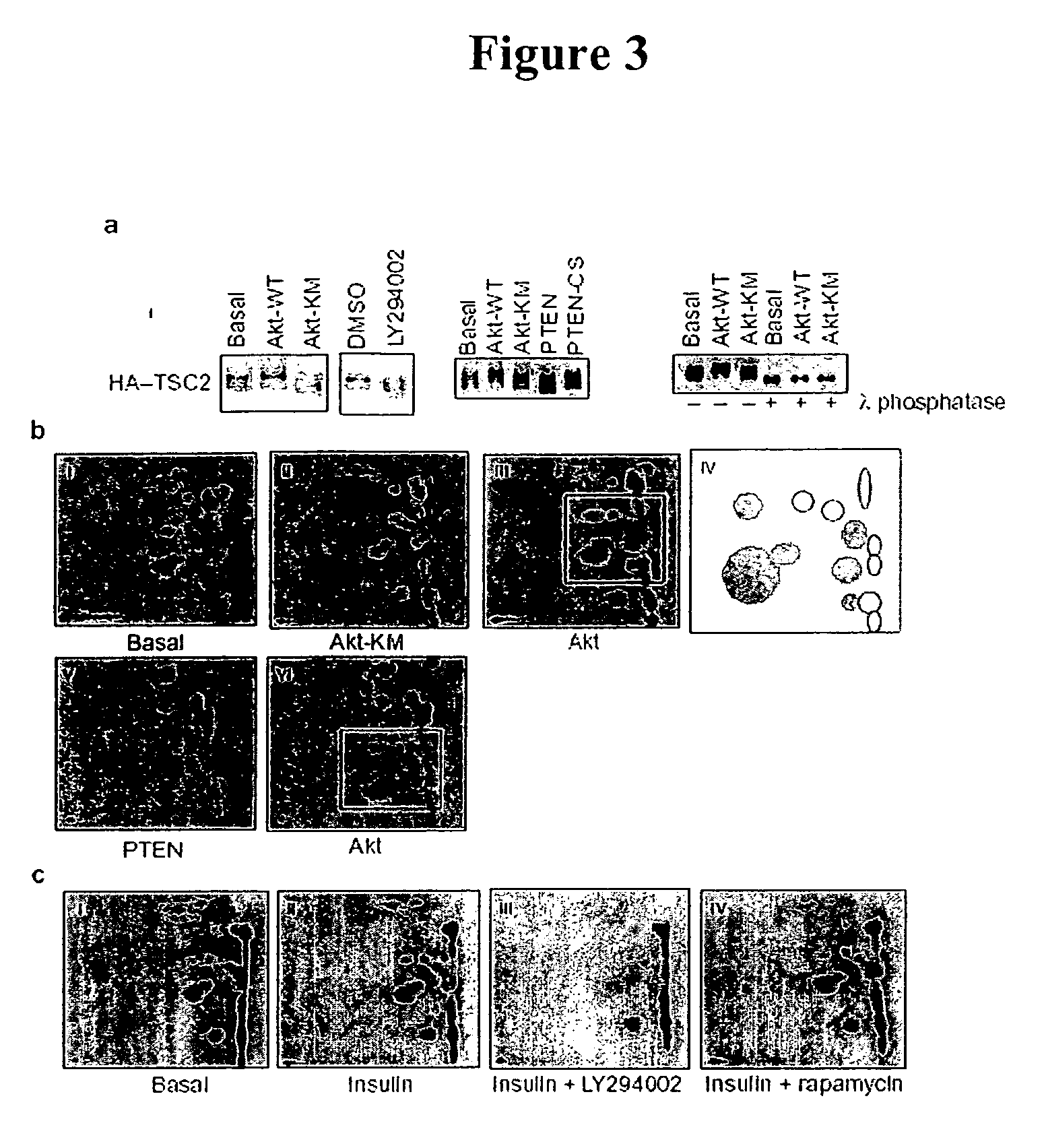
Modulating endoplasmic reticulum stress in the treatment of tuberous sclerosis
InactiveUS20100022495A1Promote apoptosisReduce and prevent ER stressOrganic active ingredientsBiocideDiseaseHAMARTOMATOUS DISEASES
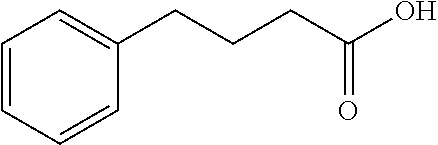
Endoplasmic reticulum stress has been found to be associated with the genetic disease tuberous sclerosis. Tuberous sclerosis is cause by defects in the two genes, TSC1 and TSC2. Agents that modulate ER stress may be used to treat tuberous sclerosis and other hamartomatous diseases. In particular, 4-phenyl butyric acid (PBA) has been shown to reduce ER stress is TSC-deficient cells. Other compounds useful in reducing ER stress are chemical chaperones such as trimethylamine N-oxide arid glycerol may also be useful in treating tuberous sclerosis. The present invention provides methods of treating a subject suffering from tuberous sclerosis using ER stress reducers such as PBA, TUDCA, UDCA, and TMAO. Methods of screening for ER stress reducers by identifying agents that reduce levels of ER stress markers in TSC-deficient cells are also provided. These agents may find use in methods and pharmaceutical compositions for treating tuberous sclerosis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Modulating Endoplasmic Reticulum Stress in the Treatment of Tuberous Sclerosis
InactiveUS20140011761A1Promote apoptosisReduce and prevent ER stressBiocideCarbohydrate active ingredientsHAMARTOMATOUS DISEASESReticulum cell
Endoplasmic reticulum stress has been found to be associated with the genetic disease tuberous sclerosis. Tuberous sclerosis is cause by defects in the two genes, TSC1 and TSC2. Agents that modulate ER stress may be used to treat tuberous sclerosis and other hamartomatous diseases. In particular, 4-phenyl butyric acid (PBA) has been shown to reduce ER stress is TSC-deficient cells. Other compounds useful in reducing ER stress are chemical chaperones such as trimethylamine N-oxide arid glycerol may also be useful in treating tuberous sclerosis. The present invention provides methods of treating a subject suffering from tuberous sclerosis using ER stress reducers such as PBA, TUDCA, UDCA, and TMAO. Methods of screening for ER stress reducers by identifying agents that reduce levels of ER stress markers in TSC-deficient cells are also provided. These agents may find use in methods and pharmaceutical compositions for treating tuberous sclerosis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Methods for the treatment or prevention of immune disorders using anti-CD40 antibodies
The present invention relates to methods and compositions for the prevention and treatment of cancer, inflammatory diseases and disorders or deficiencies of the immune system. The methods of the invention comprise, administering a CD40 binding protein that potentiates the binding of CD40 to CD40 ligand.
Owner:SEATTLE GENETICS INC
Tweak receptor
The present invention provides the TWEAK receptor and methods for identifying and using agonists and antagonists of the TWEAK receptor. In particular, the invention provides methods of screening for agonists and antagonists and for treating diseases or conditions mediated by angiogenesis, such as solid tumors and vascular deficiencies of cardiac or peripheral tissue.
Owner:IMMUNEX CORP
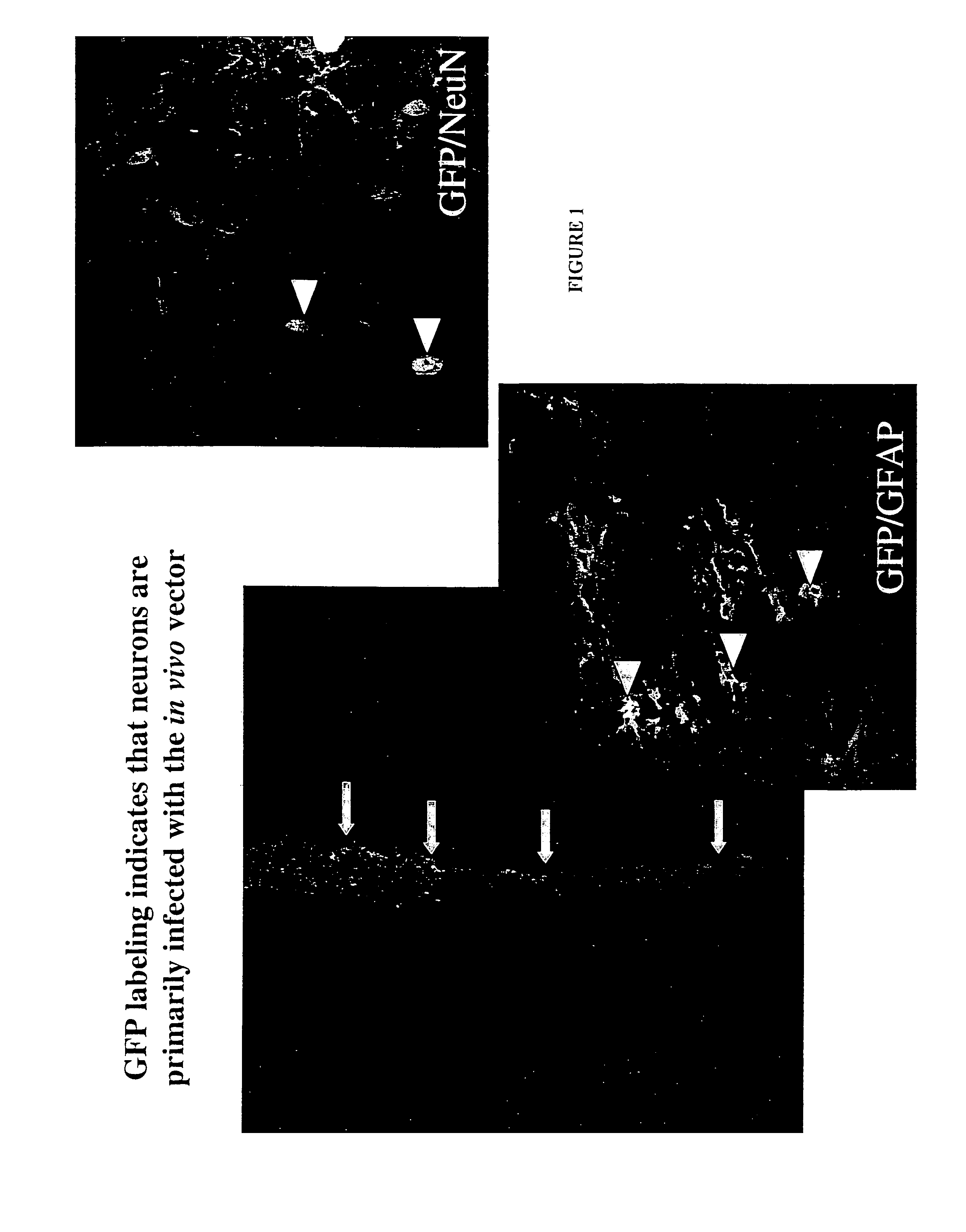
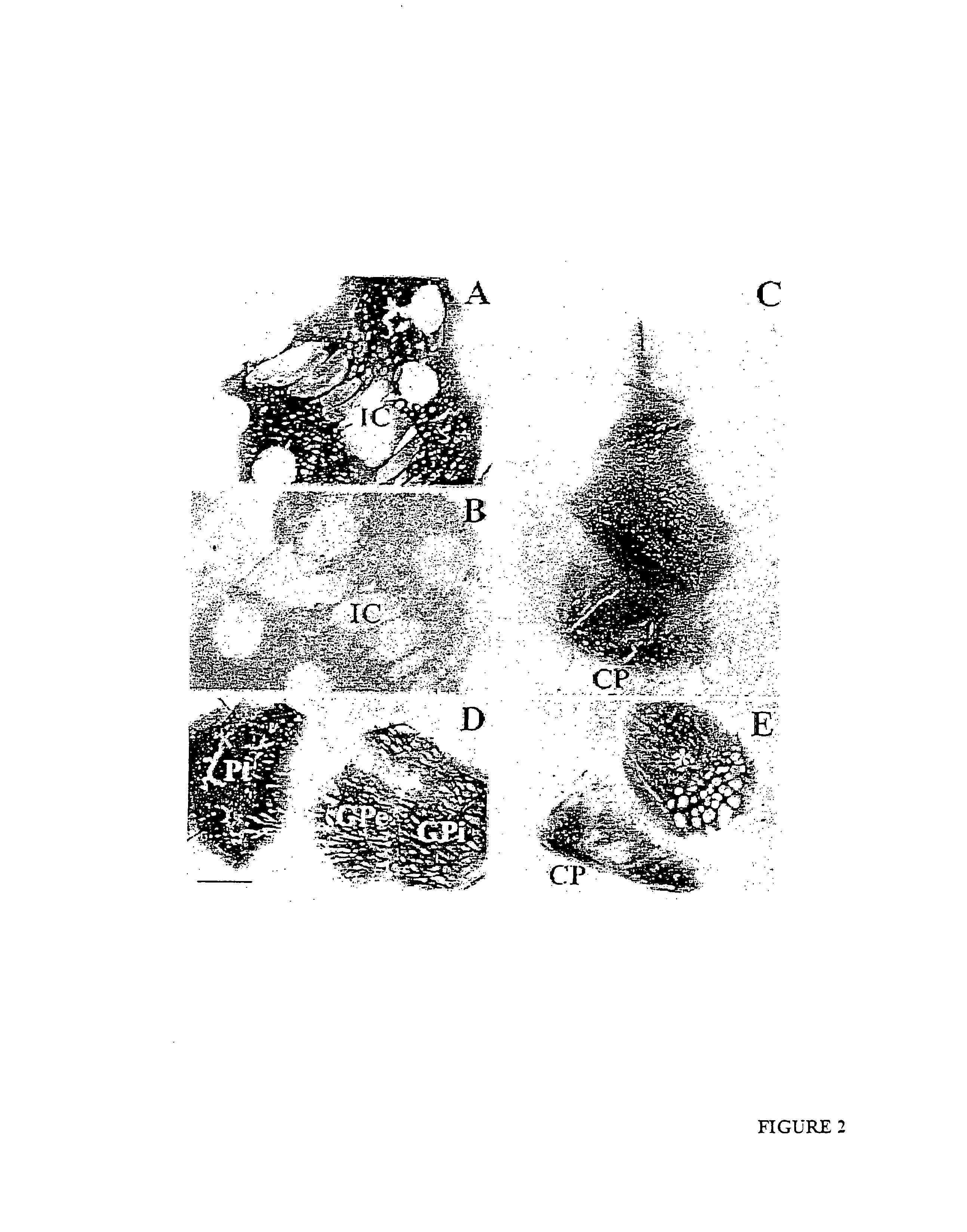
Methods for therapy of neurodegenerative disease of the brain
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 1010 to 1015 neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives from 2.5 μl to 25 μl of neurotrophic composition, delivered slowly, as in over a period of time ranging upwards of 10 minutes / delivery site. Each delivery site is at, or within 500 μm of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:RGT UNIV OF CALIFORNIA
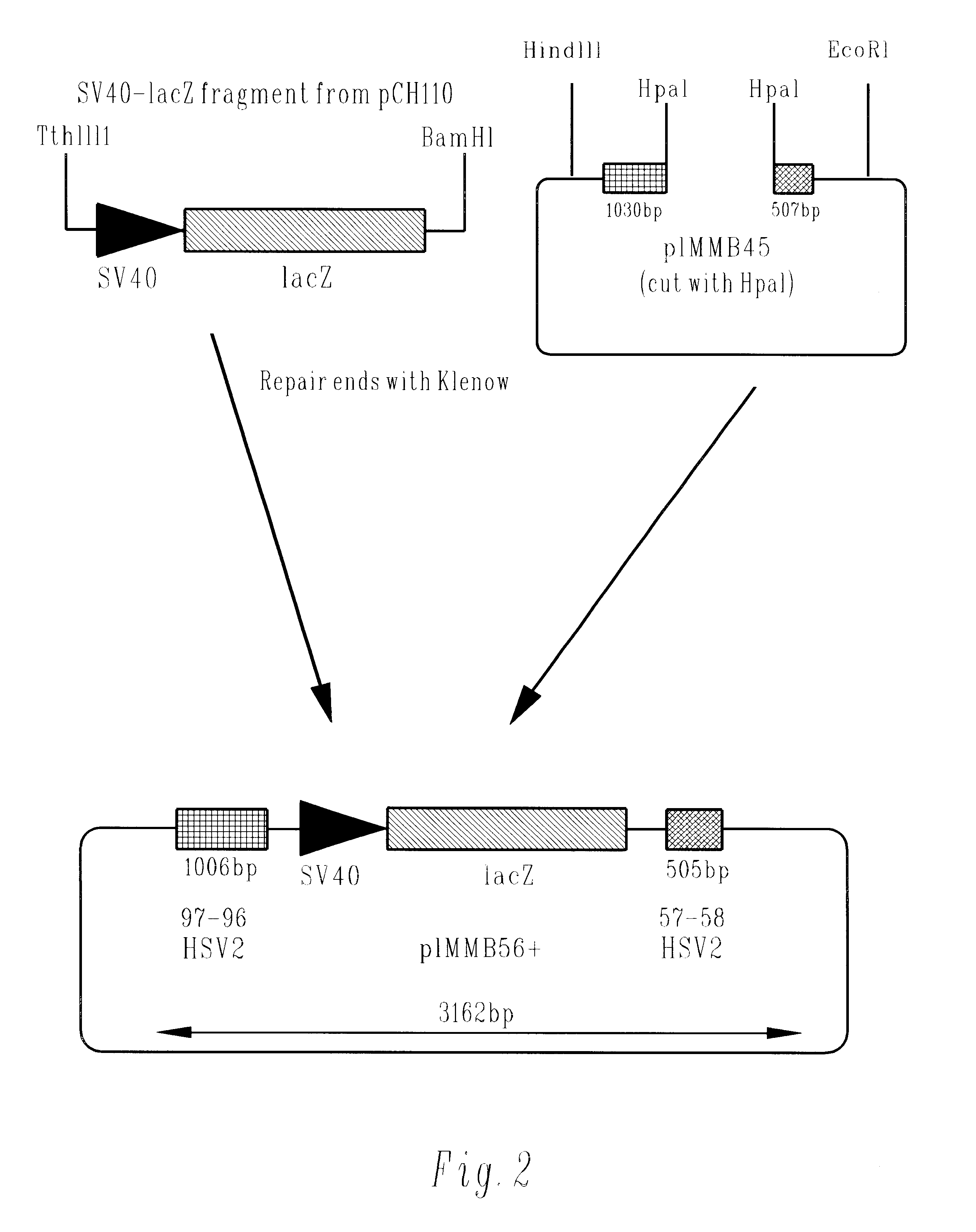
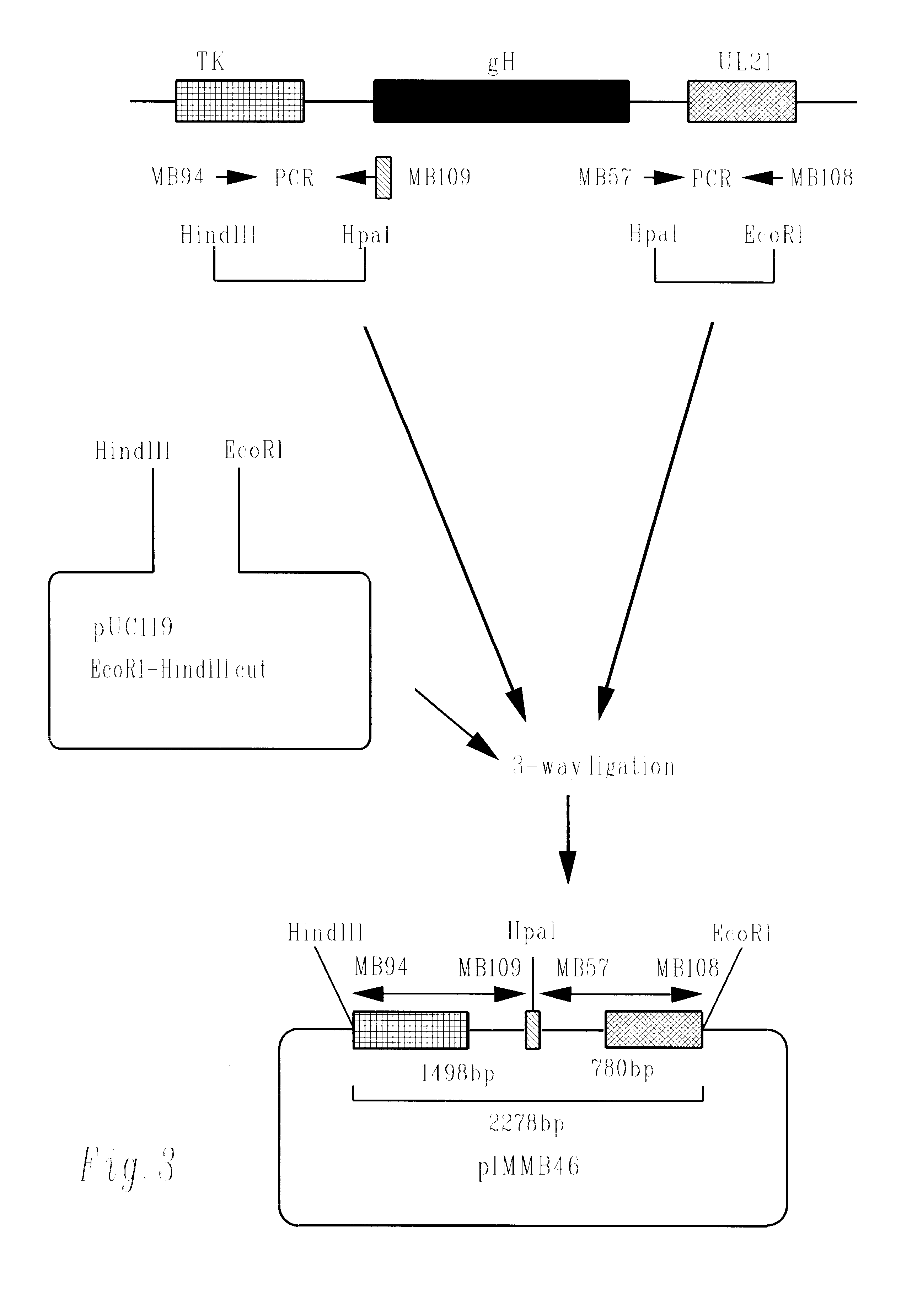
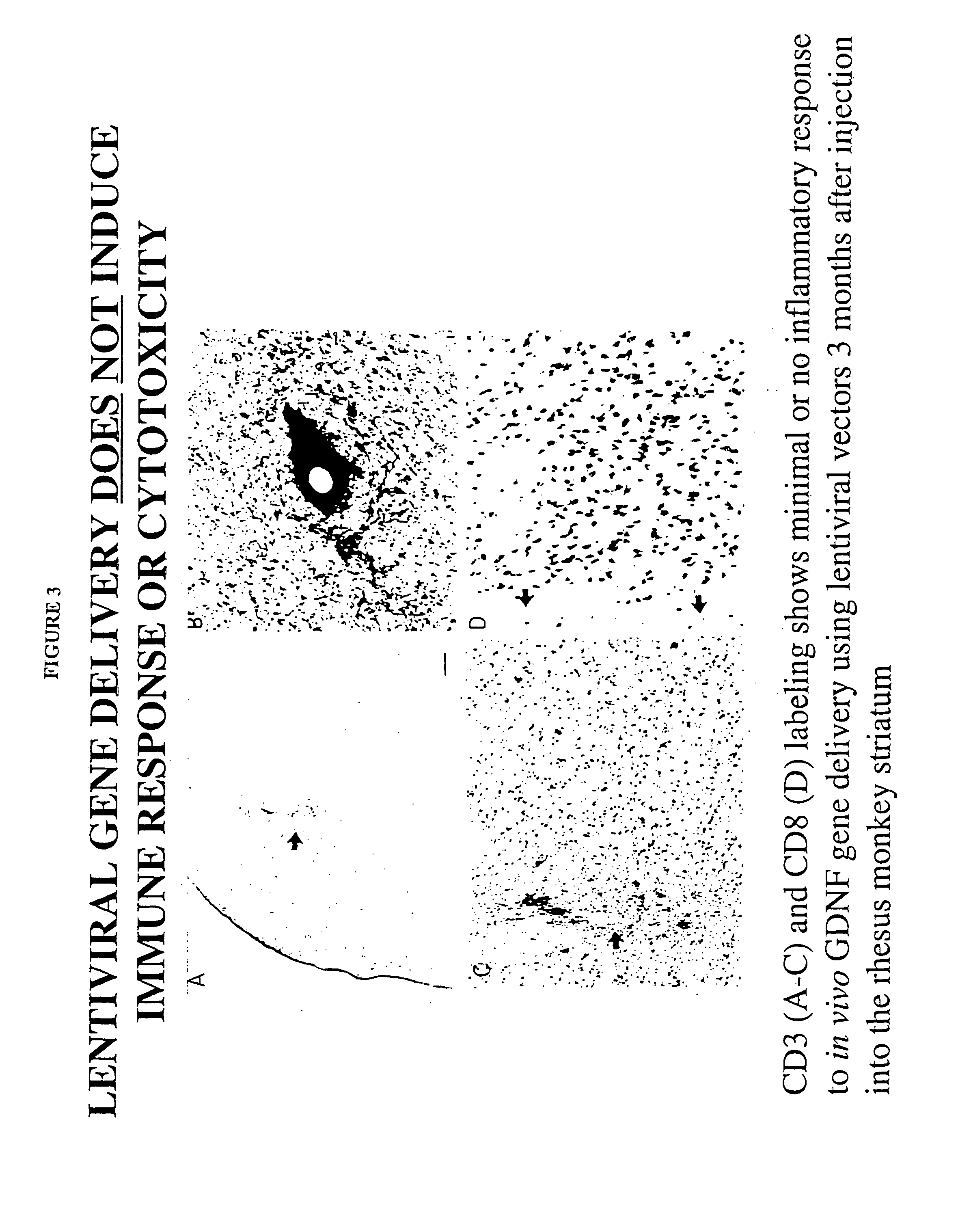
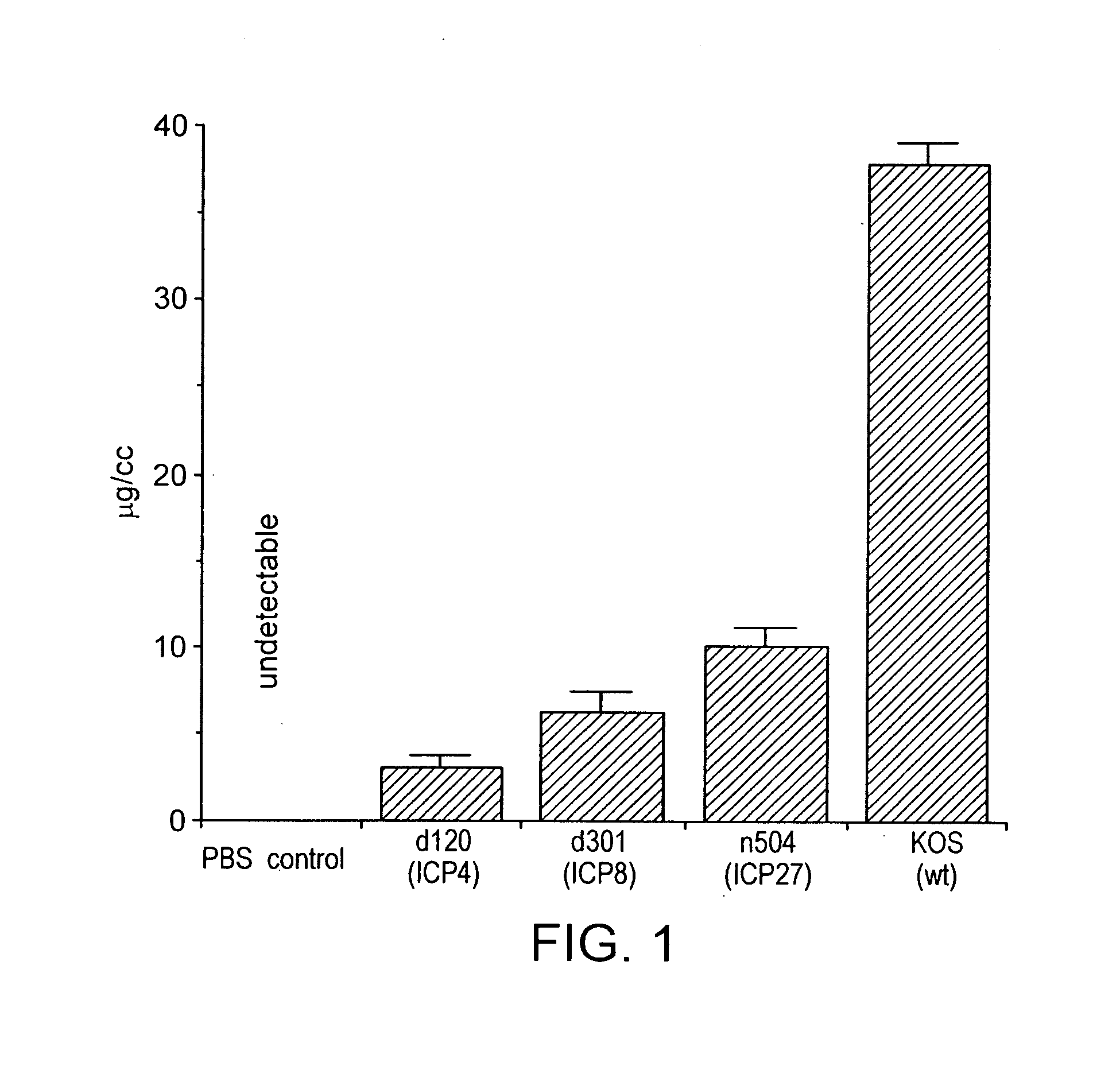
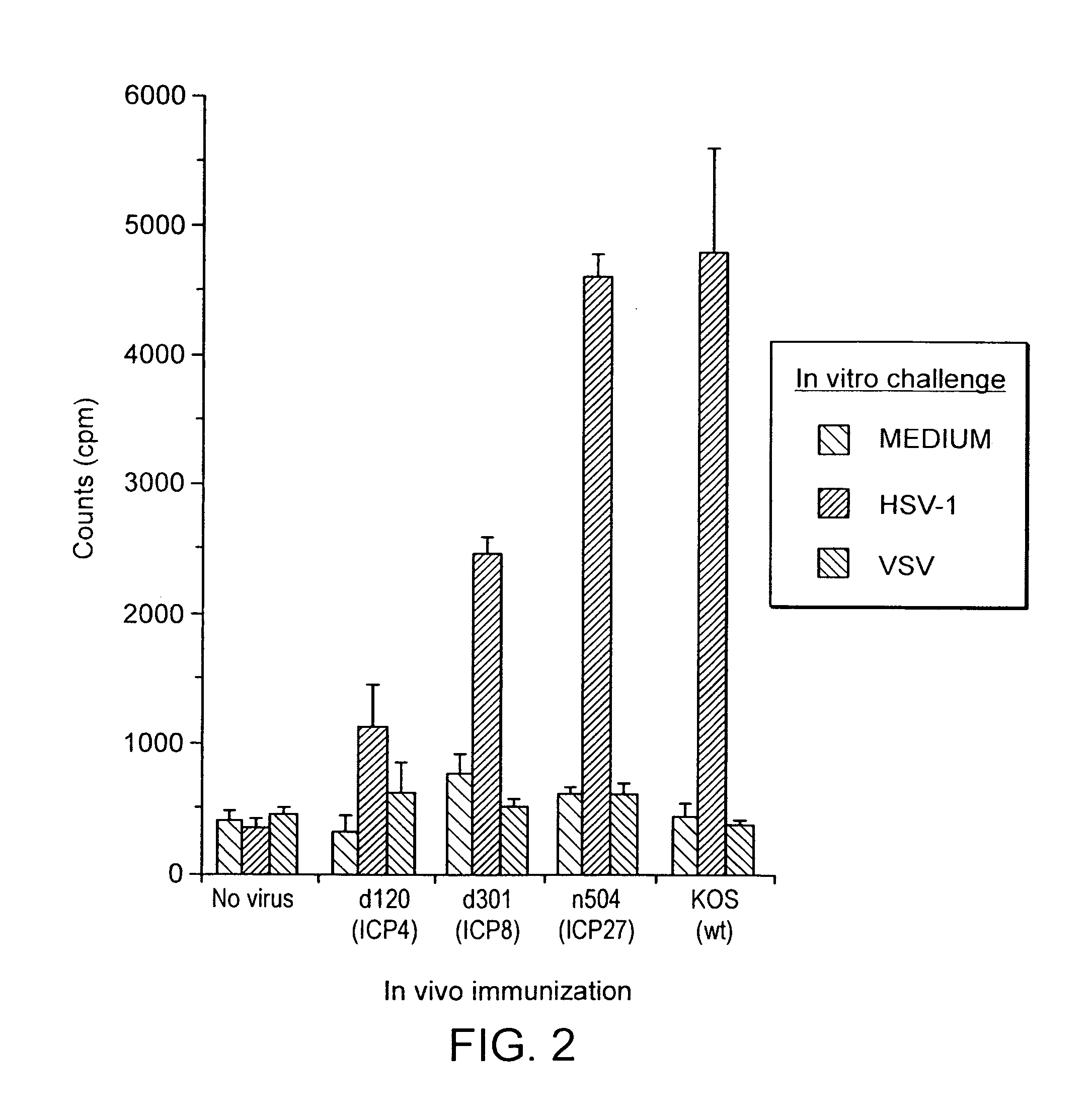
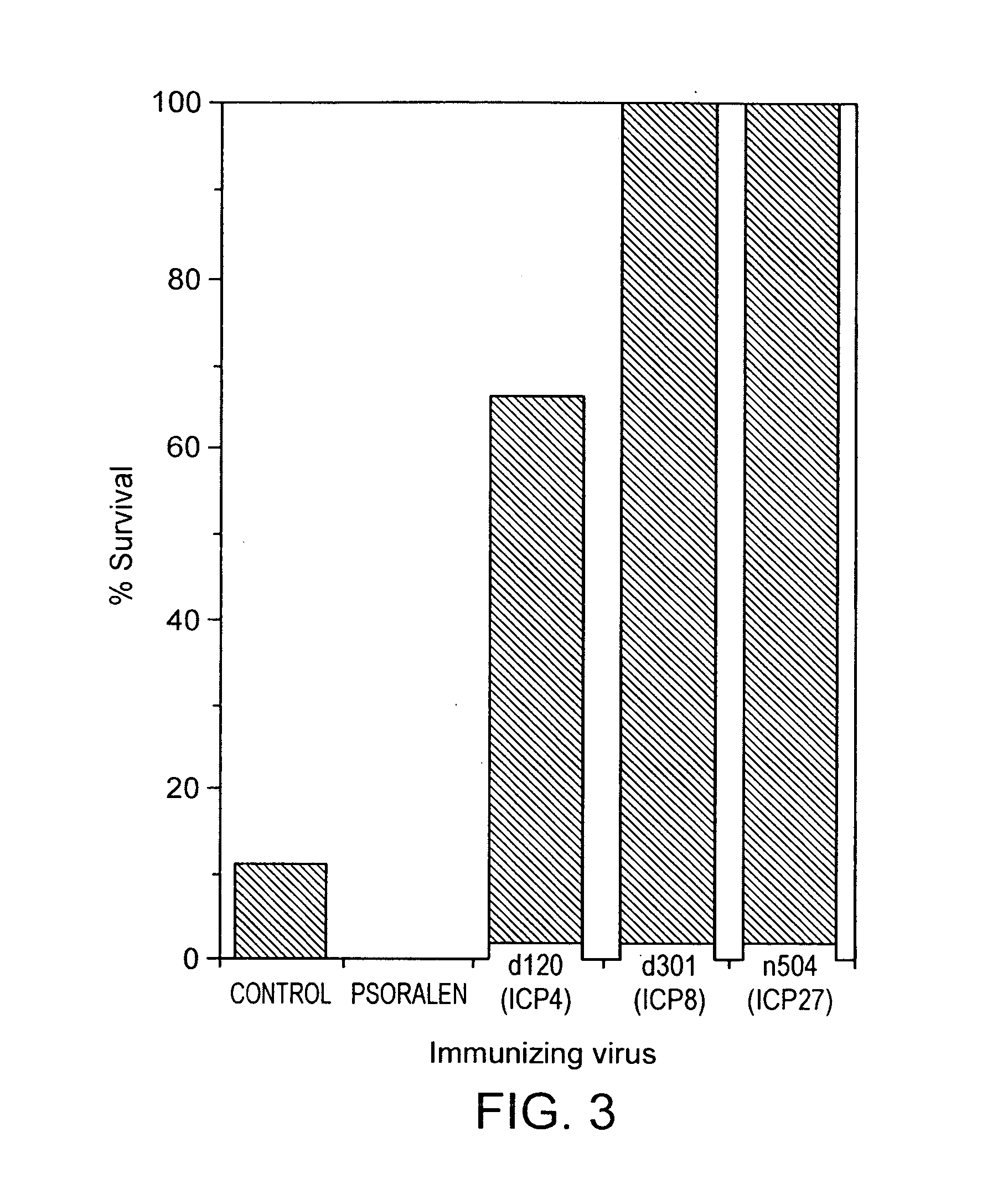
Herpesvirus replication defective mutants
ActiveUS7223411B1Avoid infectionAvoid the build processAntibody mimetics/scaffoldsViral antigen ingredientsDiseaseDefective mutant
A herpesvirus vaccine comprising a mutated herpesvirus suspended in a pharmaceutically acceptable carrier. The mutated herpesvirus is capable of infecting cells of the mammal to be vaccinated, but incapable of completing a replicative cycle, and it is capable of eliciting a protective immune response in that mammal. The mutated herpesvirus is also capable of treating immunomodulatory or immunoregulatory diseases. The mutation occurs in at least one gene encoding a protein essential for replication of the virus, so that the mutation renders the virus replication defective.
Owner:DANA FARBER CANCER INST INC +1
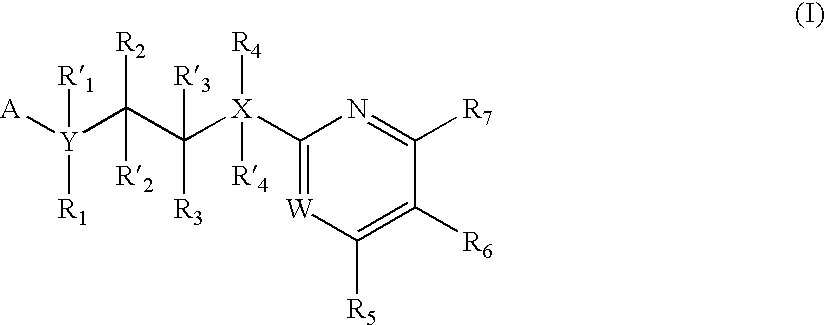
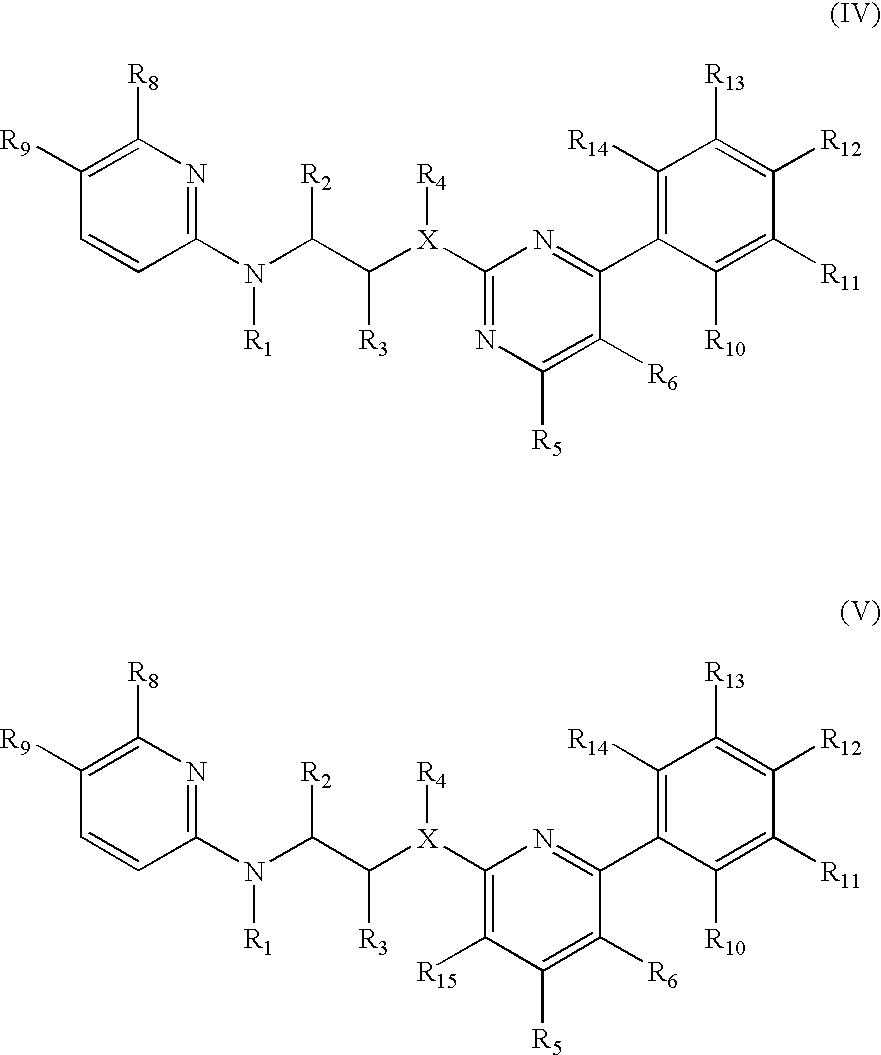
Pyrazine based inhibitors of glycogen synthase kinase 3
New bicyclic based compounds, compositions and methods of inhibiting the activity of glycogen synthase kinase (GSK3) in vitro and of treatment of GSK3 mediated disorders in vivo are provided. The methods, compounds and compositions of the invention may be employed alone, or in combination with other pharmacologically active agents in the treatment of disorders mediated by GSK3 activity, such as in the treatment of diabetes, Alzheimer's disease and other neurodegenerative disorders, obesity, atherosclerotic cardiovascular disease, essential hypertension, polycystic ovary syndrome, syndrome X, ischemia, traumatic brain injury, bipolar disorder, immunodeficiency or cancer.
Owner:CHIRON CORP
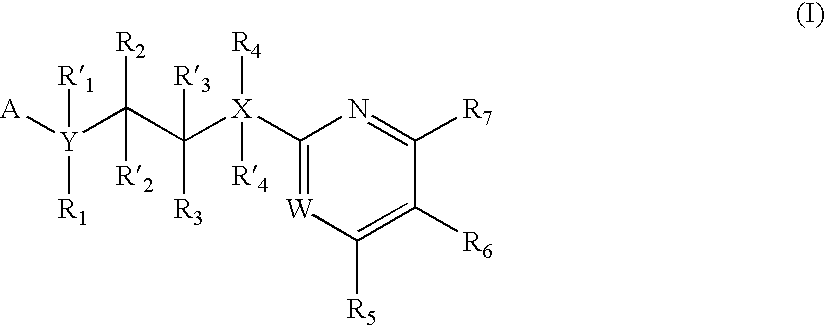
Inhibitors of glycogen synthase kinase 3
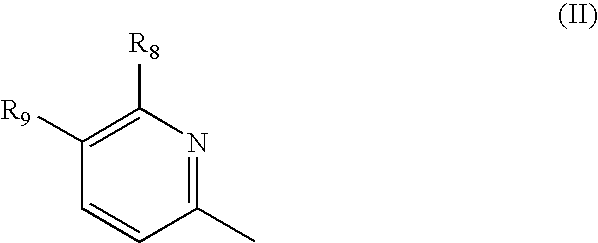
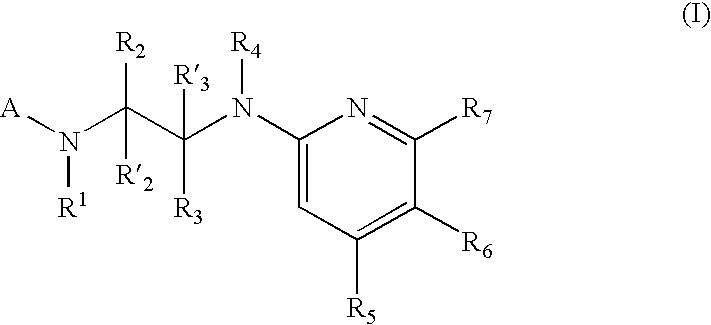
New pyridine-based compounds of Formula I, compositions, and methods of inhibiting the activity of glycogen synthase kinase (GSK3) in vitro and of treatment of GSK3-mediated disorders in vivo are provided. The methods, compounds, and compositions of the invention may be employed alone, or in combination with other pharmacologically active agents in the treatment of disorders mediated by GSK3 activity, such as diabetes, Alzheimer's disease and other neurodegenerative disorders, obesity, atherosclerotic cardiovascular disease, essential hypertension, polycystic ovary syndrome, syndrome X, ischemia, traumatic brain injury, bipolar disorder, immunodeficiency or cancer.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Lipocalin-type prostaglandin d2 synthase as a biomarker for lung cancer progression and prognosis
InactiveUS20110318308A1Small toxicityEffective treatmentOrganic active ingredientsBiocideTube formationLipocalin-type PGDS
A PGD(2) receptor (DP) deficiency enhances tumor progression accompanied by abnormal vascular expansion. In tumors, angiogenic endothelial cells highly express DP receptor, and its deficiency accelerates vascular leakage and angiogenesis. Administration of a synthetic DP agonist, BW245C, markedly suppresses tumor growth as well as tumor hyperpermeability in WT mice, but not in DP-deficient mice. In a corneal angiogenesis assay and a modified Miles assay, host DP deficiency potentiates angiogenesis and vascular hyperpermeability under COX-2-active situation, whereas exogenous administration of BW245C strongly inhibits both angiogenic properties in WT mice. In an in vitro assay, BW245C does not affect endothelial migration and tube formation, processes that are necessary for angiogenesis; however, it strongly improves endothelial barrier function via an increase in intracellular cAMP production. PGD(2) / DP receptor is a newly identified regulator of tumor vascular permeability, indicating DP agonism can be exploited as a therapy for the treatment of cancer.
Owner:WINTHROP UNIV HOSPITAL
TWEAK receptor
The present invention provides the TWEAK receptor and methods for identifying and using agonists and antagonists of the TWEAK receptor. In particular, the invention provides methods of screening for agonists and antagonists and for treating diseases or conditions mediated by angiogenesis, such as solid tumors and vascular deficiencies of cardiac or peripheral tissue.
Owner:IMMUNEX CORP
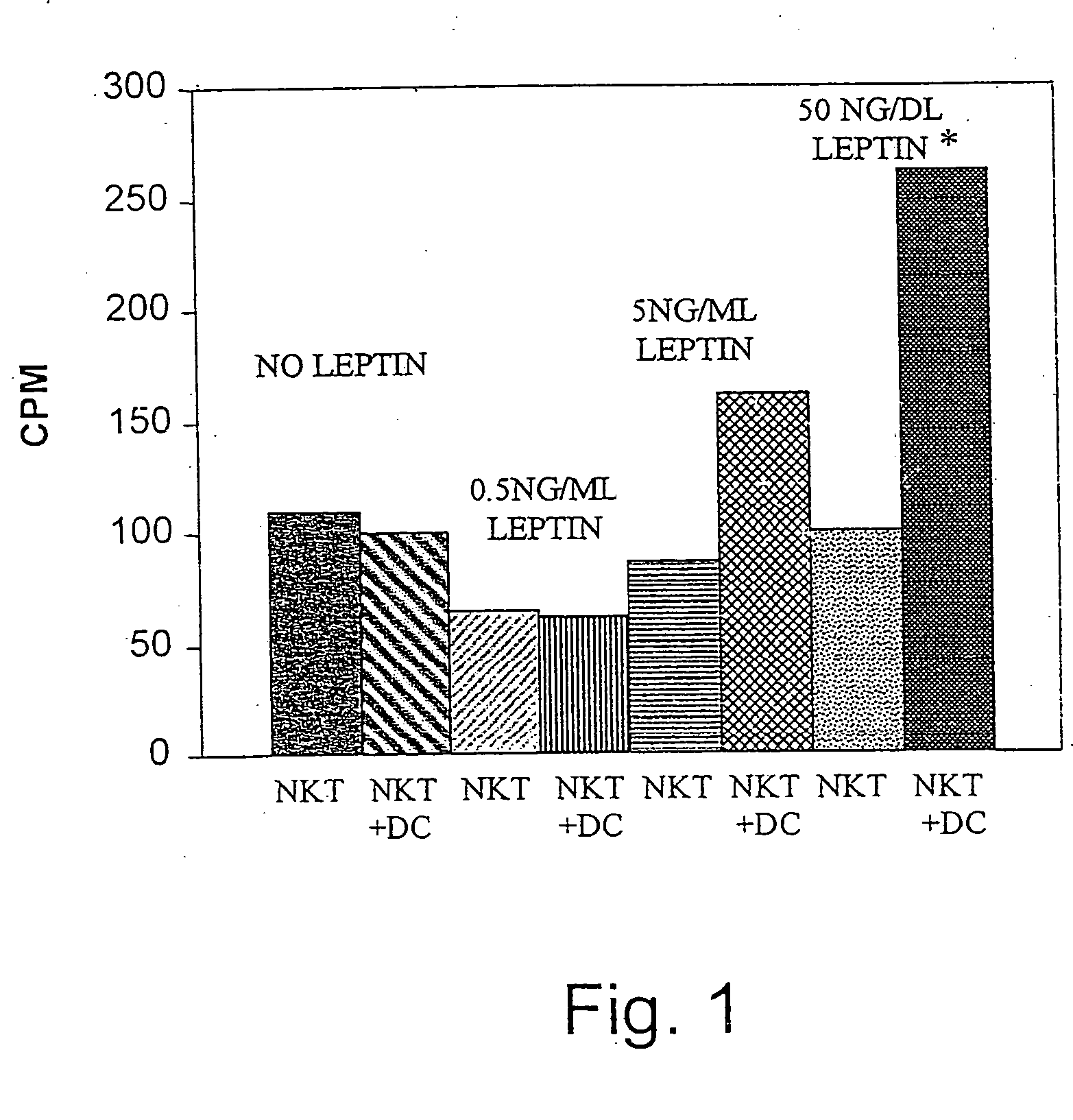
Methods and uses of leptin in immune modulation and hepatocellular carcinoma
Leptin was previously demonstrated to exert potent immune modulatory properties in several immune mediated disorders. The aim of the study was to determine leptin's anti-tumor effect in a murine model of human hepatocellular carcinoma (HCC). In vivo, Athymic T cell deficient (nude) mice transplanted with 1×106 human Hep3B cells, followed by administration of two daily intraperitoneal doses of 0.5 mg / gram leptin for 6 weeks. Leptin administration induced a significant reduction in tumor size and improved survival in nude mice. Histologically, tumors of leptin-administered mice featured increased inflammatory exudate in interphase areas. Leptin-induced tumor suppression was associated with a significant increase in peripheral natural killer (NK) cell number. Splenocytes from leptin-treated mice featured decreased expression of CIS mRNA. To determine which lymphocyte subset is a prerequisite for the anti tumor effect of leptin, T&B cell deficient (Scid) mice and T,B& NK deficient (Scid-Beige) mice were subcutaneously implanted with Hep3B tumor cells, with and without the daily intraperitoneal administration of 0.5 mg / gram leptin for 6 weeks. SCID mice featured leptin-associated tumor suppression similar to those of nude mice. In contrast, NK-deficient SCID-Beige mice developed larger tumors. To further establish natural killer cell's central role in mediation of leptin's anti-tumor effect, NK cells were incubated in vitro with increasing doses of leptin, demonstrating a dose-dependent increase in cytotoxic activity. Incubation of leptin with hepatoma cell line was found to induce a dose-dependent reduction in hepatoma cell proliferation, suggesting an additive direct anti-tumor effect. Further synergism in inhibition of hepatoma cell proliferation in vitro was achieved following addition of natural killer cells. HCC cells expressed leptin receptor mRNA, while addition of leptin induced increased mRMA expression of STAT2 and SOCS1 on tumor cell lines. Leptin administration induces a significant suppression of human HCC. This effect is mediated by induction of natural killer cell proliferation and activation, and by direct inhibition of tumor growth. Decreased natural killer cell expression of inhibitory CIS protein and over expression of the anti-proliferative STAT2 and SOCS1 proteins in HCC lines may underline both anti cancerous effects of leptin.
Owner:ENZO THERAPEUTICS
Methods and reagents for modulating macrophage phenotype
ActiveUS20190290688A1Restore immune recognitionReduced polarization effectsOrganic active ingredientsPeptide/protein ingredientsP PHENOTYPENeonatal Fc receptor
The present invention is directed to methods of inducing a phenotypic change in a population of monocytes and / or macrophages. The method includes administering to the population of monocytes and / or macrophages, a macrophage stimulating agent coupled to a carrier molecule, wherein the carrier molecule facilitates macropinocytic uptake of the agent by monocytes and macrophages in the population and is defective in neonatal Fc receptor binding, wherein the administering induces a phenotypic change in the monocytes and macrophages in the population.
Owner:NEW YORK UNIV
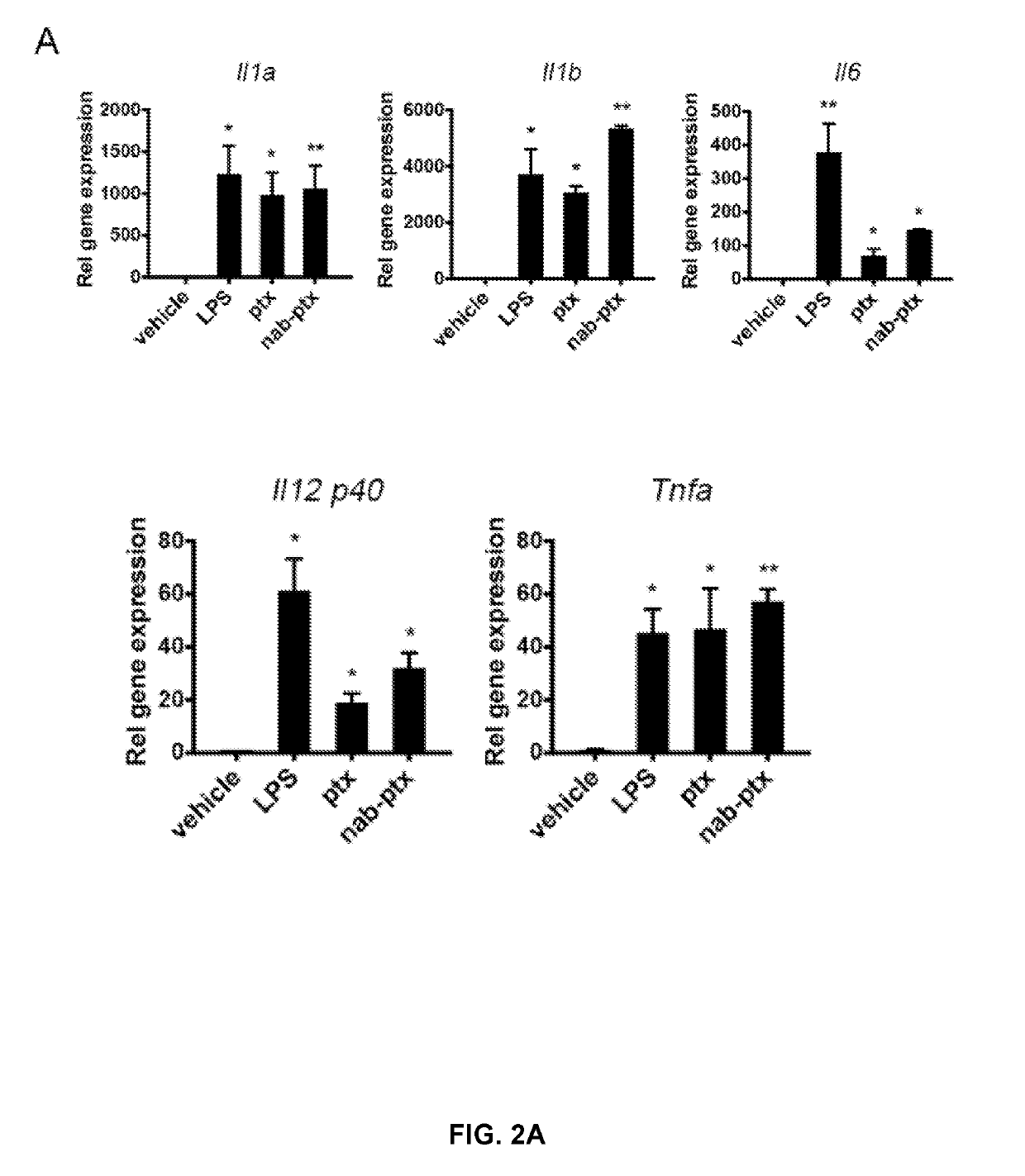
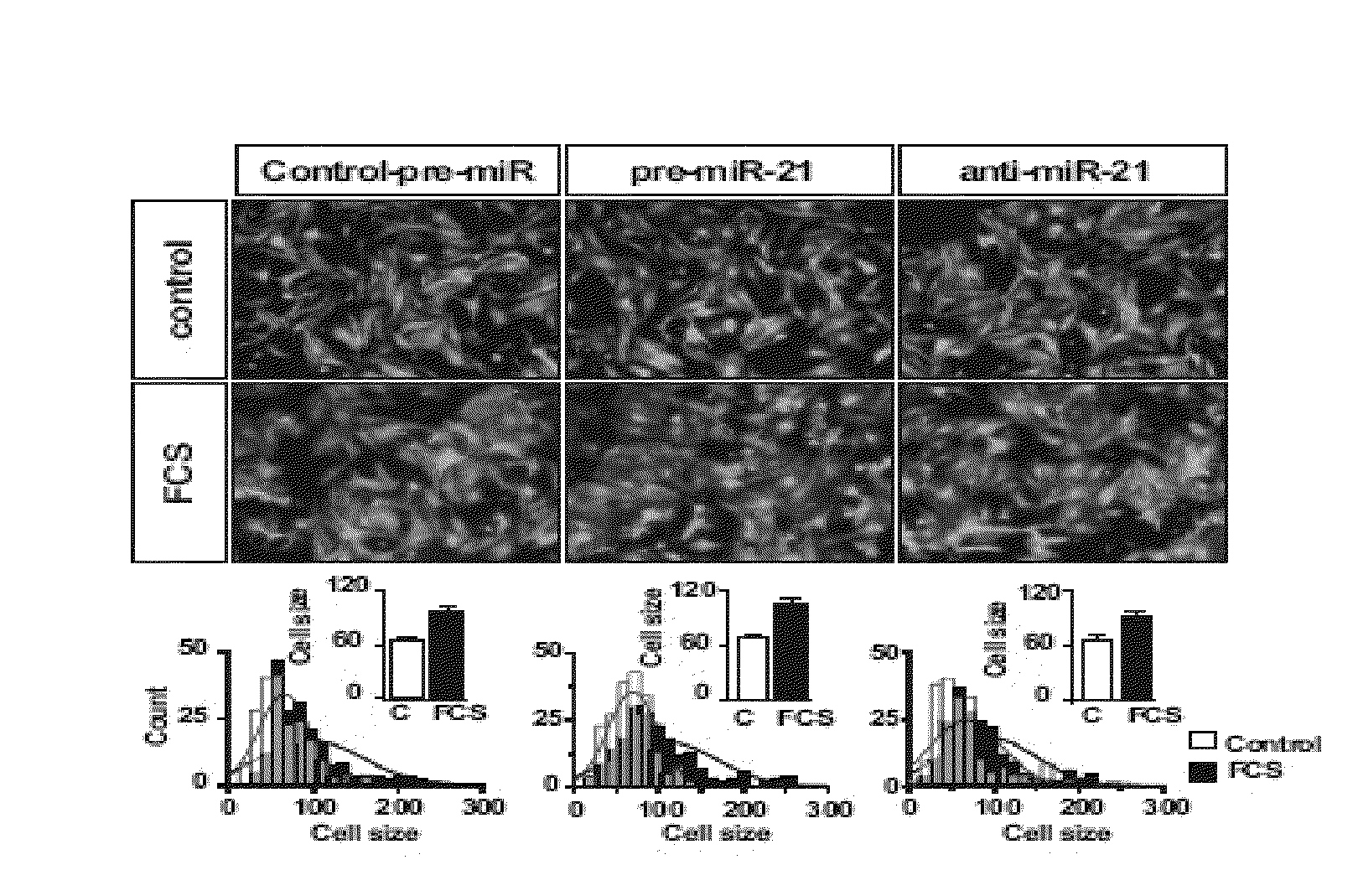
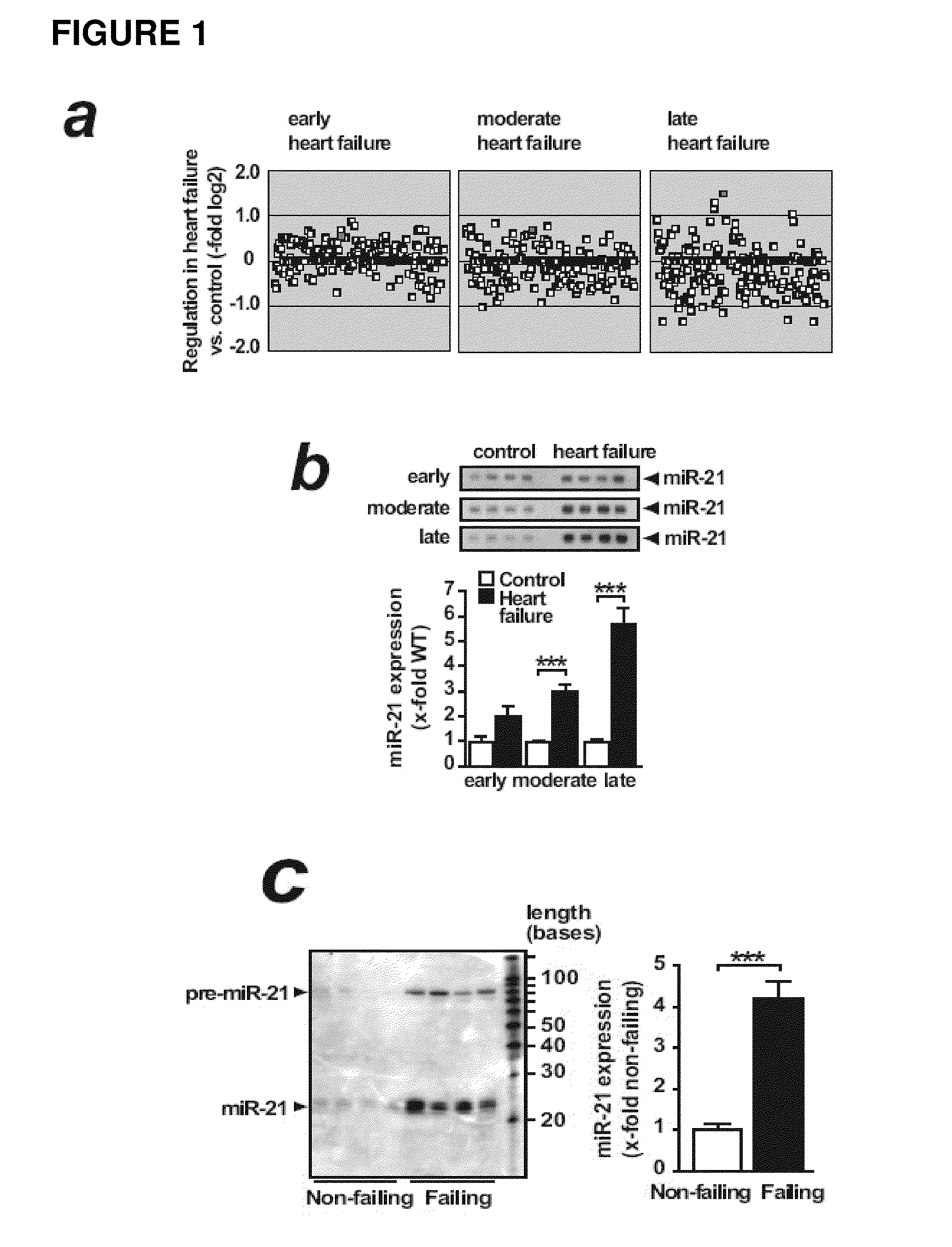
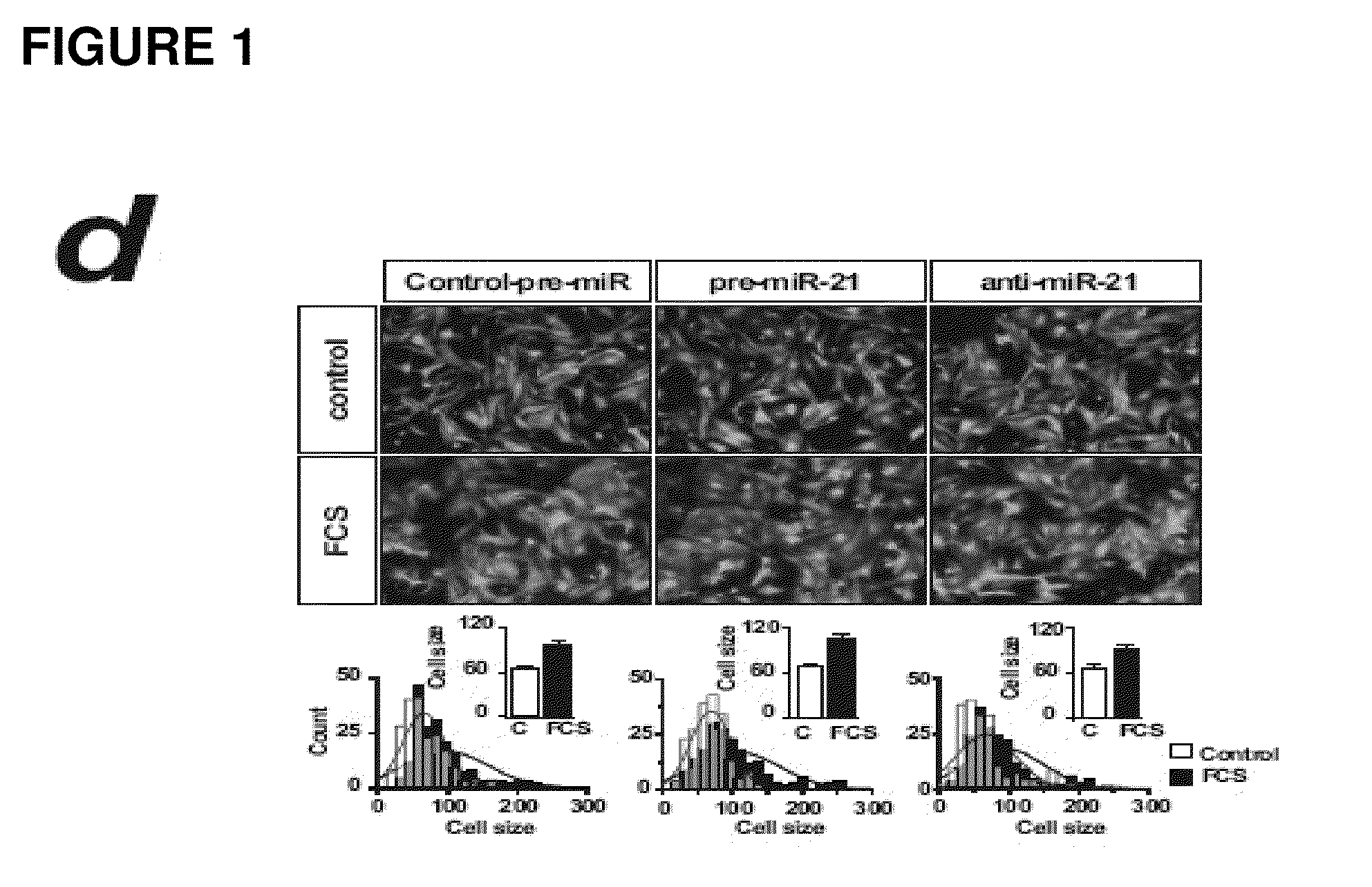
MicroRNA (miRNA) And Downstream Targets For Diagnostic And Therapeutic Purposes
ActiveUS20110071211A1Prevents heart weight increase and leftMitigation of impairmentAntipyreticGenetic material ingredientsDiseaseFibrosis
The present invention relates to a promoter region of a microRNA, the use of a microRNA, in particular miR-21, and related elements for the diagnosis and for the manufacture of a medicament for the treatment and / or prevention of fibrosis and / or fibrosis related diseases. Additionally, the invention concerns antisense oligonucleotides against targets of miR-21. A cell deficient for miR-21, the promoter region and targets of miR-21 and a knock-out organism thereof are also encompassed. Finally, the invention is directed to a method for diagnosing fibrosis and / or fibrosis related diseases and to a method for screening a pharmaceutically active compound for the treatment of fibrosis and / or fibrosis related diseases. The present invention further relates to compositions for use in the treatment, amelioration, and / or prevention of fibrosis. In certain embodiments, the compositions modulate the activity of a miRNA for the treatment, amelioration, and / or prevention of fibrosis. In certain embodiments, the compositions inhibit the activity of miR-21 for the treatment, amelioration, and / or prevention of fibrosis.
Owner:JULIUS MAXIMILIANS UNIV WURZBURG
Inhibitors of glycogen synthase kinase 3
New pyrimidine or pyridine based compounds, compositions and methods of inhibiting the activity of glycogen synthase kinase (GSK3) in vitro and of treatment of GSK3 mediated disorders in vivo are provided. The methods, compounds and compositions of the invention may be employed alone, or in combination with other pharmacologically active agents in the treatment of disorders mediated by GSK3 activity, such as diabetes, Alzheimer's disease and other neurodegenerative disorders, obesity, atherosclerotic cardiovascular disease, essential hypertension, polycystic ovary syndrome, syndrome X, ischemia, traumatic brain injury, bipolar disorder, immunodeficiency or cancer.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
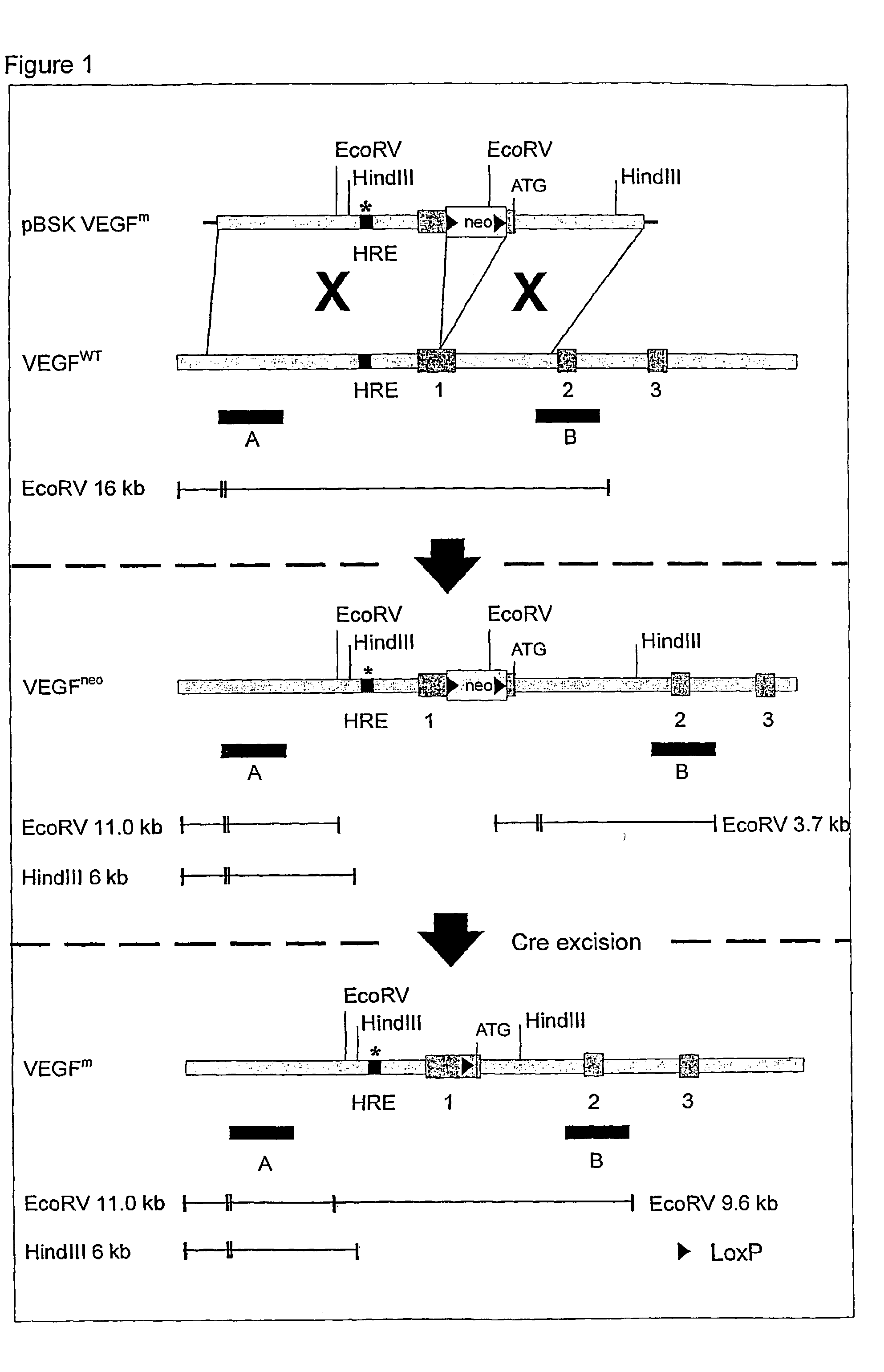
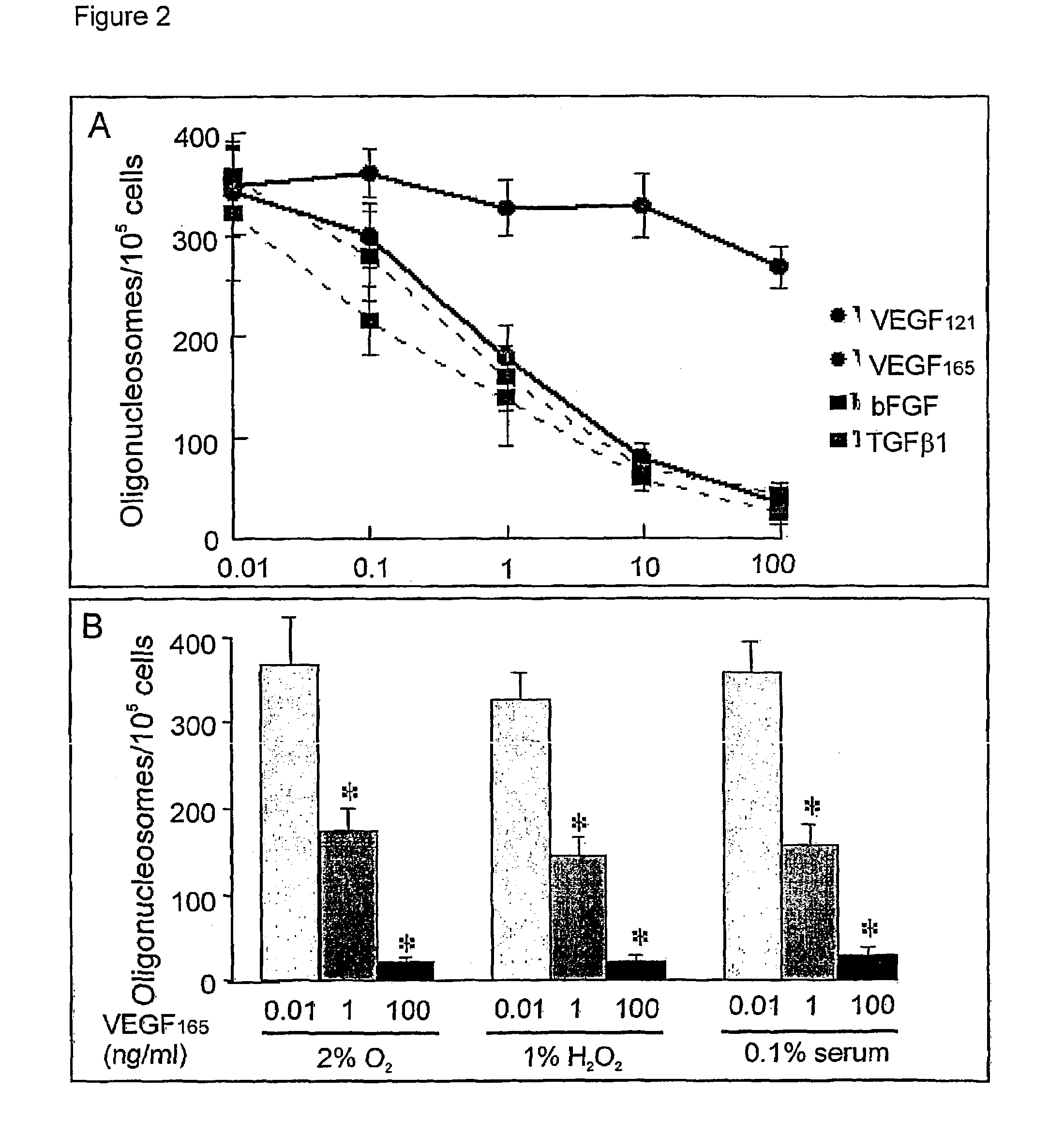
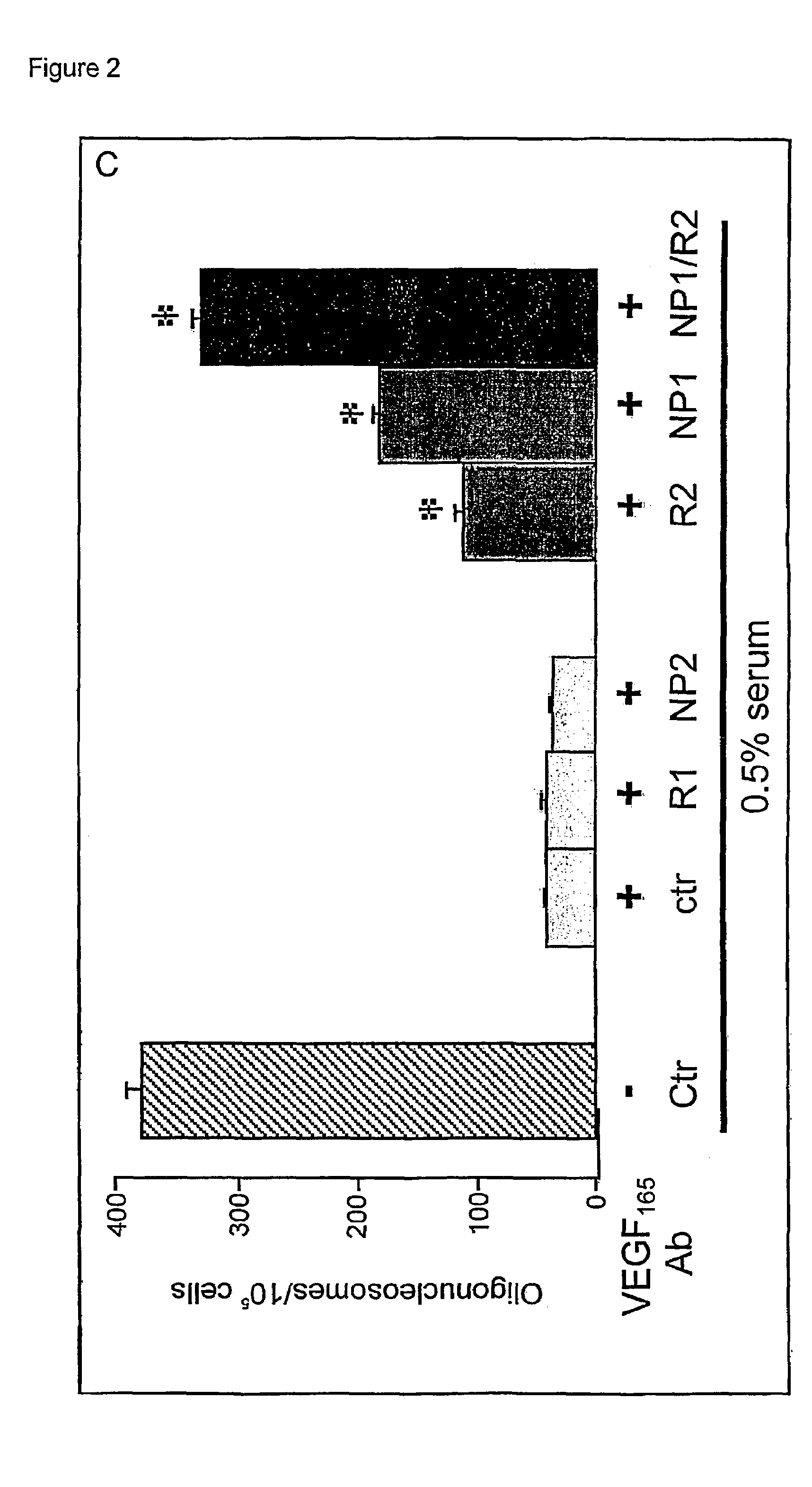
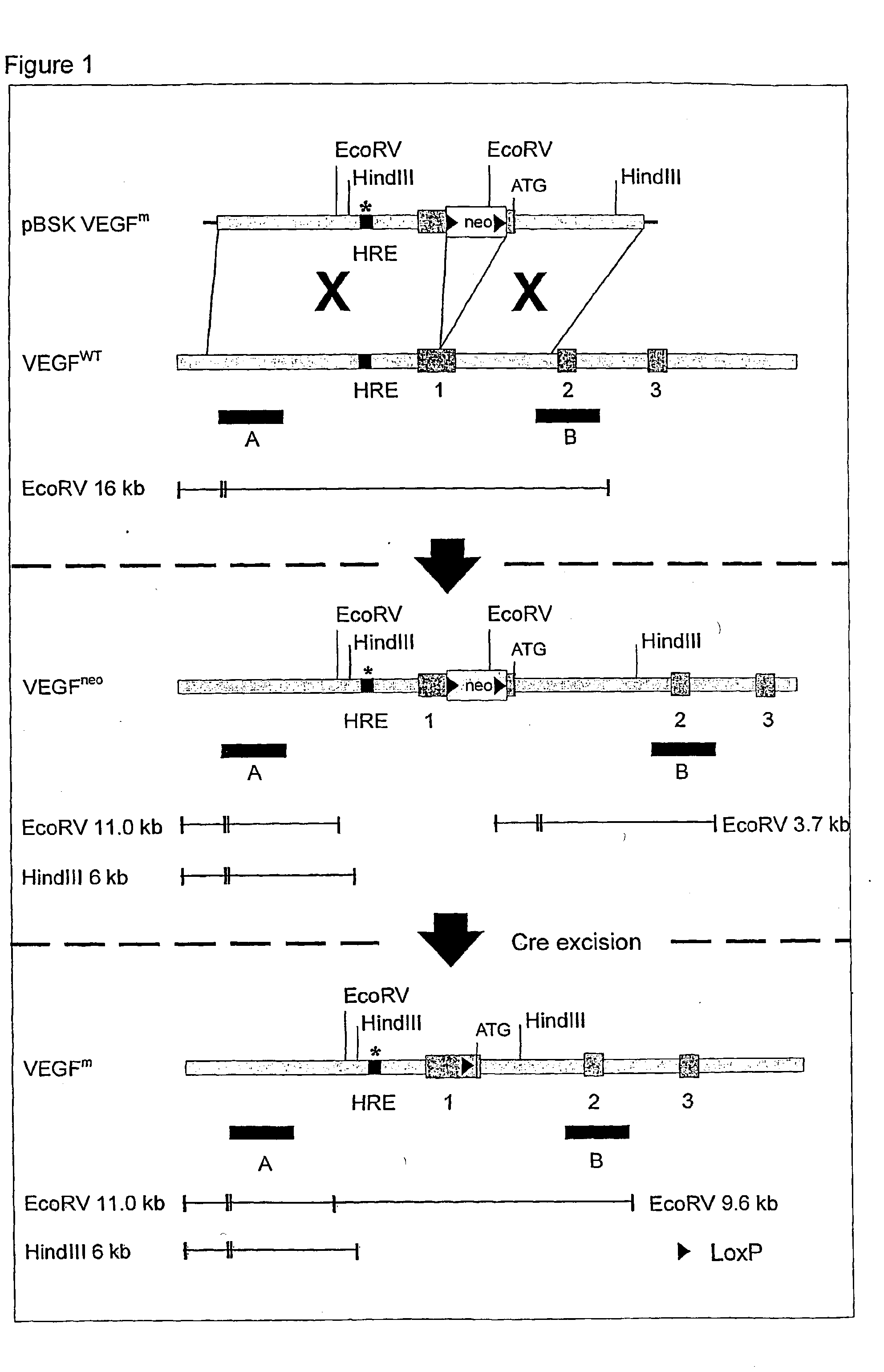
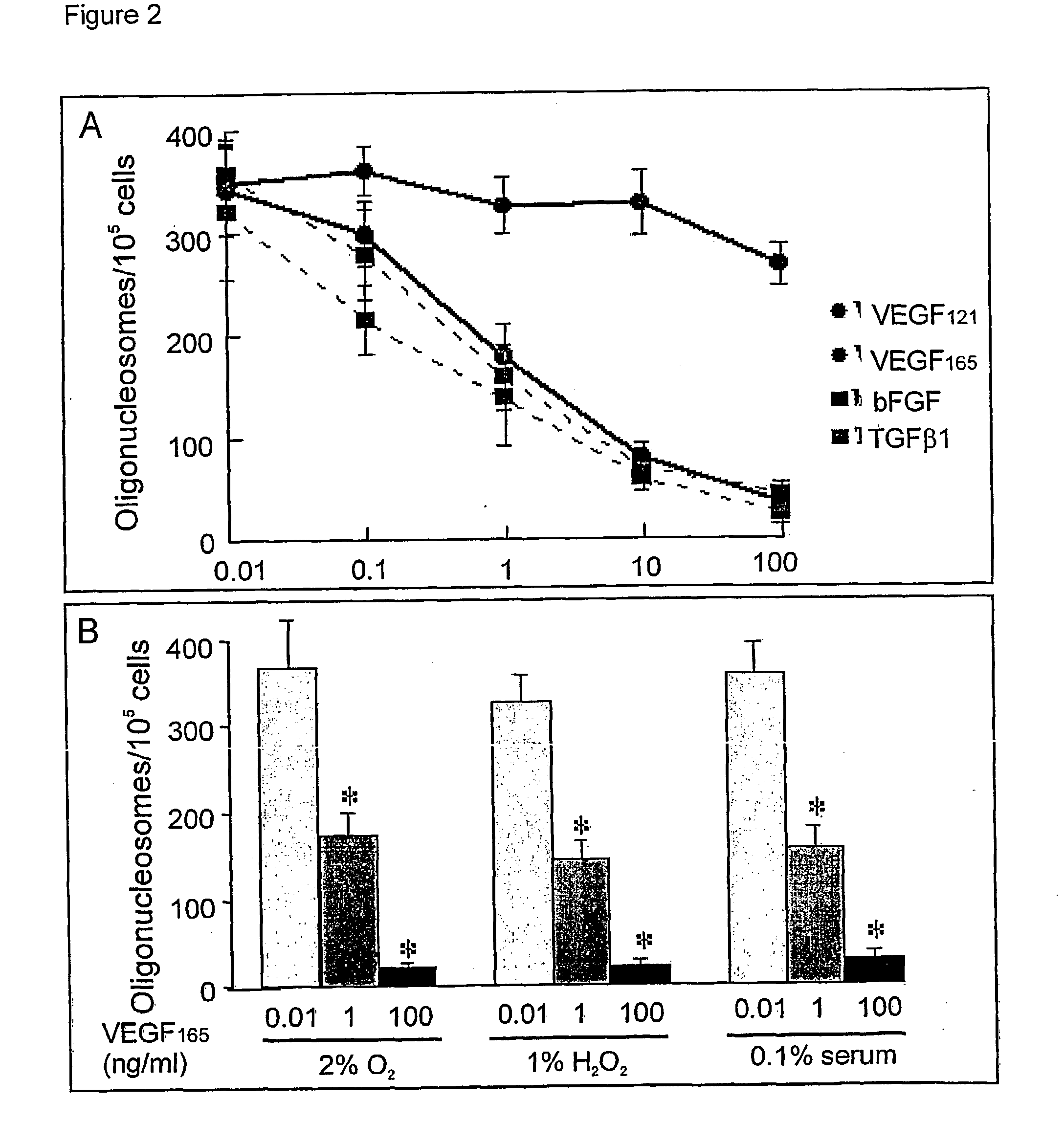
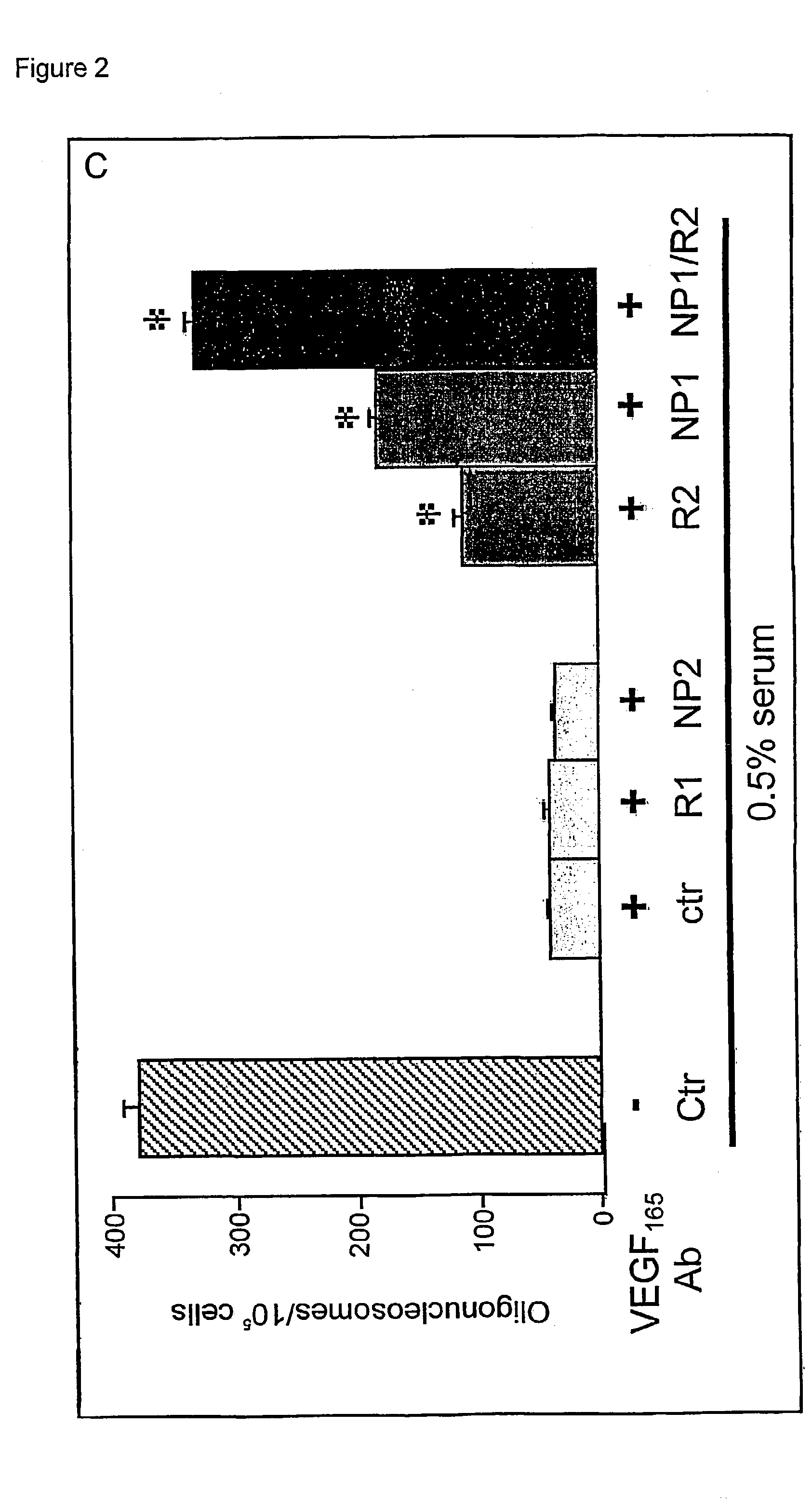
Use of VEGF and homologues to treat neuron disorders
InactiveUS7226908B2Nervous disorderPeptide/protein ingredientsTruncal muscle weaknessSurvival of motor neuron
The present invention relates to neurological and physiological dysfunction associated with neuron disorders. In (particular, the invention relates to the involvement of vascular endothelial growth factor (VEGF) and homologues in the aetiology of motor neuron disorders. The invention further concerns a novel, mutant transgenic mouse (VEGFm / m) with a homozygous deletion in the hypoxia responsive element (HRE) of the VEGF promoter which alters the hypoxic upregulation of VEGF. These mice suffer severe adult onset muscle weakness due to progressive spinal motor neuron degeneration which is reminiscent of amyotrophic lateral sclerosis (ALS)—a fatal disorder with unknown aetiology. Furthermore, the neuropathy of these mice is not caused by vascular defects, but is due to defective VEGF-mediated survival signals to motor neurons. The present invention relates in particular to the isoform VEGF165 which stimulates survival of motor neurons via binding to neuropilin-1, a receptor known to bind semaphorin-3A which is implicated in axon retraction and neuronal death, and the VEGF Receptor-2. The present invention thus relates to the usage of VEGF, in particular VEGF165, for the treatment of neuron disorders and relates, in addition, to the usage of polymorphisms in the VEGF promotor for diagnosing the latter disorders.
Owner:LIFE SCI RES PARTNERS VZW +1
Diagnosis and treatment of diseases arising from defects in the tuberous sclerosis pathway
The present invention relates to compositions and methods for identifying abnormalities in TSC signaling pathways. In particular, the present invention relates to methods of diagnosing and treating disorders such as tuberous sclerosis, which are caused by mutations in the TSC genes. The present invention further relates to methods and compositions for treating cancers mediated by TSC signaling disorders.
Owner:RGT UNIV OF MICHIGAN
Use of vegf and homologues to treat neuron disorders
InactiveUS20030105018A1Impaired hypoxic upregulationDeterioration progressNervous disorderPeptide/protein ingredientsTruncal muscle weaknessSurvival of motor neuron
The present invention relates to neurological and physiological dysfunction associated with neuron disorders. In (particular, the invention relates to the involvement of vascular endothelial growth factor (VEGF) and homologues in the aetiology of motor neuron disorders. The invention further concerns a novel, mutant transgenic mouse (VEGFm / m) with a homozygous deletion in the hypoxia responsive element (HRE) of the VEGF promoter which alters the hypoxic upregulation of VEGF. These mice suffer severe adult onset muscle weakness due to progressive spinal motor neuron degeneration which is reminiscent of amyotrophic lateral sclerosis (ALS)-a fatal disorder with unknown aetiology. Furthermore, the neuropathy of these mice is not caused by vascular defects, but is due to defective VEGF-mediated survival signals to motor neurons. The present invention relates in particular to the isoform VEGF165 which stimulates survival of motor neurons via binding to neuropilin-1, a receptor known to bind semaphorin-3A which is implicated in axon retraction and neuronal death, and the VEGF Receptor-2. The present invention thus relates to the usage of VEGF, in particular VEGF165, for the treatment of neuron disorders and relates, in addition, to the usage of polymorphisms in the VEGF promotor for diagnosing the latter disorders.
Owner:LIFE SCI RES PARTNERS VZW +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com