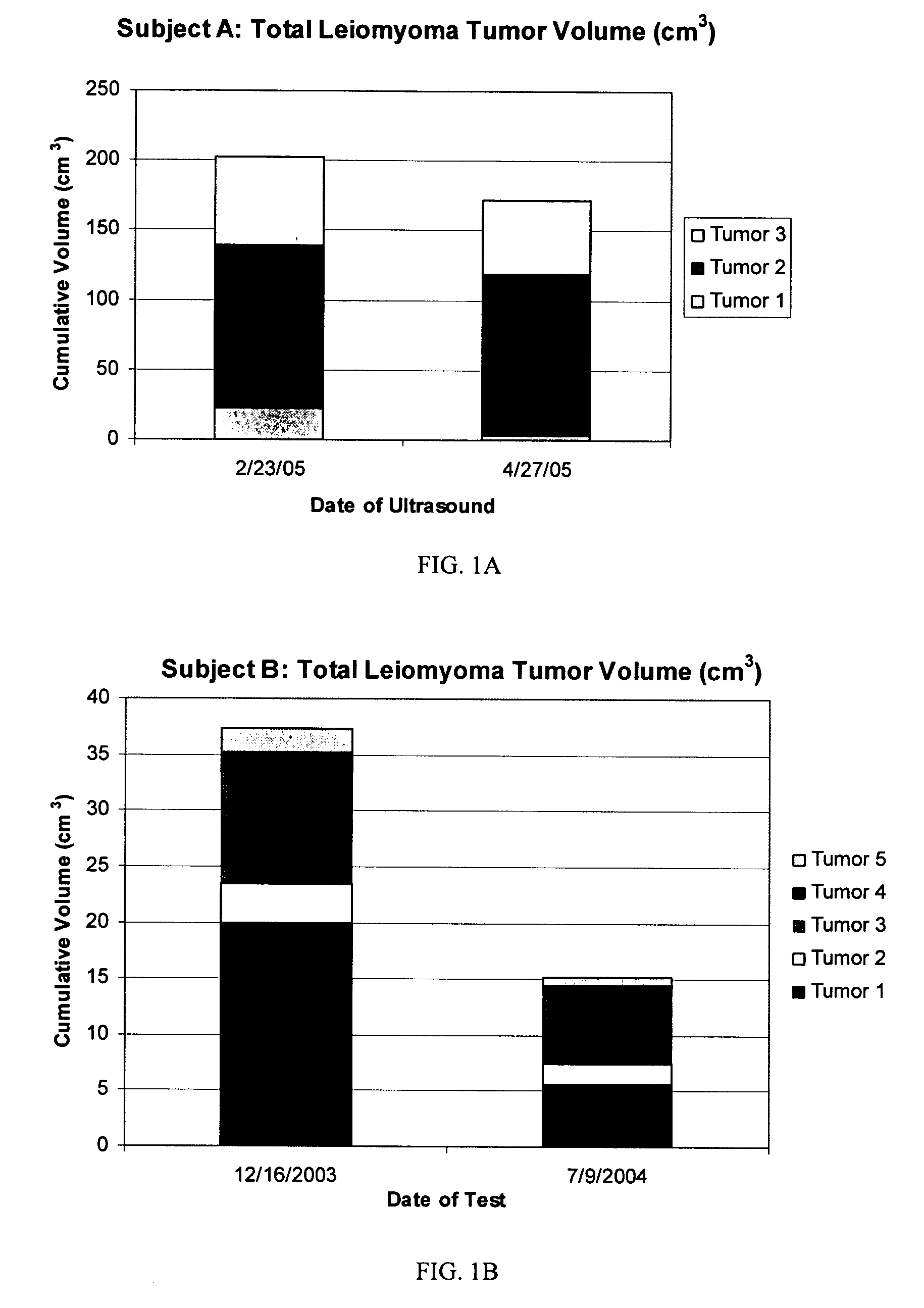
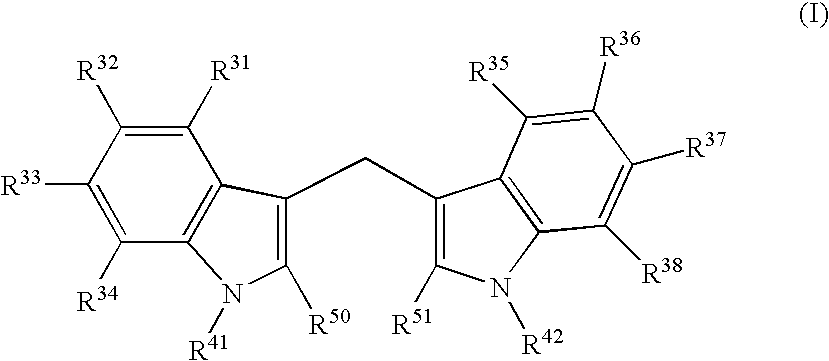
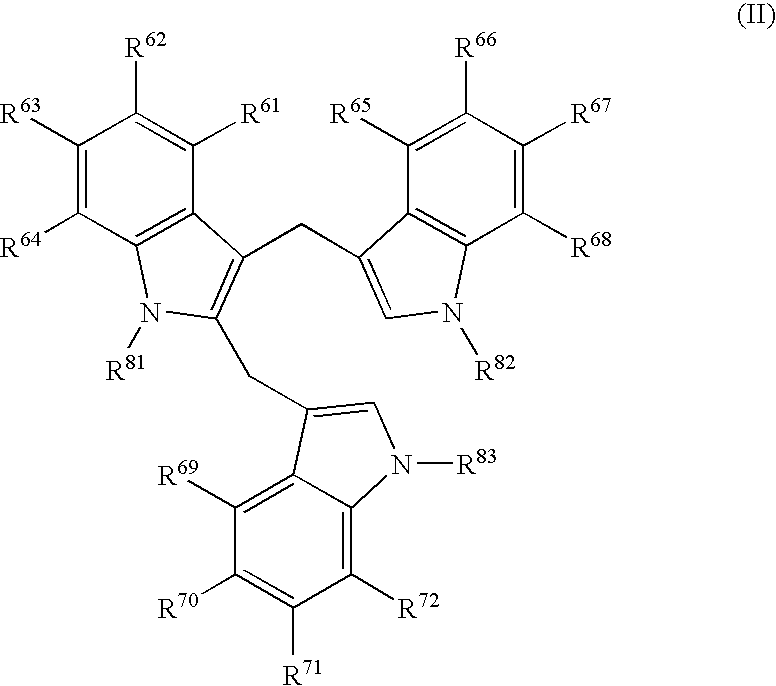
Diindolylmethane formulations for the treatment of leiomyomas
a technology of diindolylmethane and formulations, which is applied in the field of diindolylmethane formulations for the treatment of leiomyomas, can solve the problems of pelvic discomfort, bowel and bladder dysfunction, and the pathobiology of leiomyomas remains poorly understood, and achieves the effect of increasing the efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6. EXAMPLE 1
Manufacture of an Absorption-Enhanced Formulation of Dim for Treated of Leiomyomas
[0120] In general, a suitable (therapeutically effective) amount of diindolylmethane is preferably administered in an absorption enhancing formulation, as described in U.S. Pat. No. 6,086,915, at 100-2000 mg per day as a suspension of microparticles in a starch carrier matrix. The actually administered amounts of Diindolylmethane may be decided by a supervising physician.
[0121] Preparation of processed Diindolylmethane was accomplished according to the steps outlined in U.S. Pat. No. 6,086,915, herein incorporated by reference in its entirety. Briefly, this included mixture of about 10-40% by final weight of either diindolylmethane with about 10-40% by final weight of vitamin E polyethylene glycol 1000 succinate (Vitamin-E-TPGS, Eastman Chemical), 2-20% by final weight, phosphatidyl choline (Phospholipon 50G, Rhone Poulenc) and 15-30% by final weight hexanol. This mixture was made homogen...
example 2
7. EXAMPLE 2
Manufacture of Capsules Containing Diindolylmethane
[0123] Capsules containing 150-300 mg of processed diindolylmethane, as produced according to the steps described in Example 1, were made by mixing the processed diindolylmethane with microcrystaline cellulose and placing the mixed powder into opaque gelatin capsules.
[0124] Alternatively, capsules containing processed diindolylmethane (DIM), as produced according to the steps described in Example 1, were made by mixing the processed DIM with polyphenolic EGFR inhibitory compounds according to the following per capsule amounts. The ingredients are mixed according to these amounts and capsules are filled using standard machinery.
TABLE 5Preferred CapsuleRange of CapsuleComponentWeight (mg)Weight (mg)Processed DIM 75 (50-100)Silibinin (SiliPhos)100(100-200)Green Tea Extract (EGCG)150(150-300)Total Active Fill Weight (mg)325(300-625)
[0125] Optionally, 6-25 mg of Lycopene is added to each capsule and / or 25-100 mg of Scutel...
example 3
8. EXAMPLE 3
Manufacture of a Vaginal Suppository Formulation of Dime for Treatment of Leiomyomas in Conjuction with Oral Therapy
[0126] In a heated vessel, 90 grams cetostearyl alcohol (Alfol 16 / 18, Vista) was heated to 100° C. to which 5 gms of microcrystalline DIM (LKT Labs, St. Paul, Minn.) was added with constant mixing to form a hot slurry. Alternatively, 90 grams cetostearyl alcohol (Alfol 16 / 18, Vista) is heated to 100° C. to which 5 gms of microcrystalline DIM is mixed, alone or together with 10 grams of ceramide or synthetic cerimide derivatives, C2 ceramide. In a second vessel 400 gms of IV Novata (Semi-synthetic Glyceride Suppository Base, Ashland Chemicals) was warmed to 40° C. with constant mixing. The well mixed slurry from the first vessel was added with continued mixing to the second vessel. The homogenized molted suppository material was formed into suppositories of 2 gms each and cooled. Glyceryl monsterate 10-50 gms was added to the molten mixture as needed to inc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com