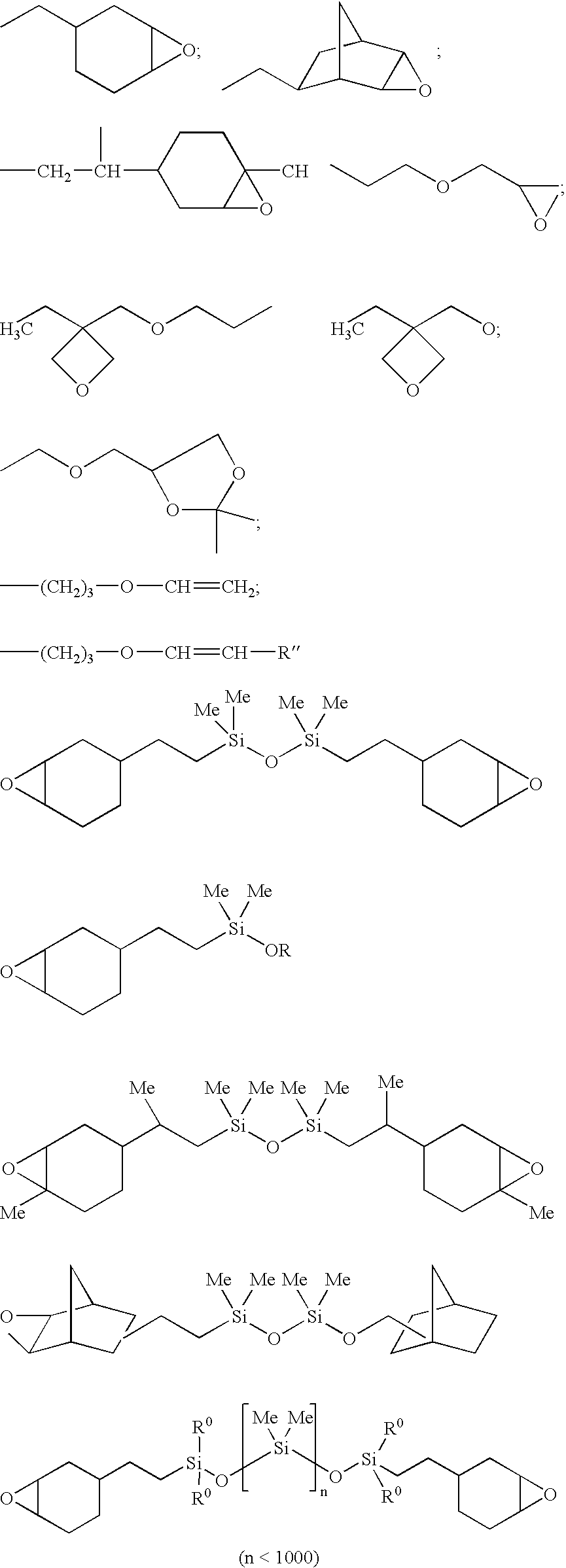
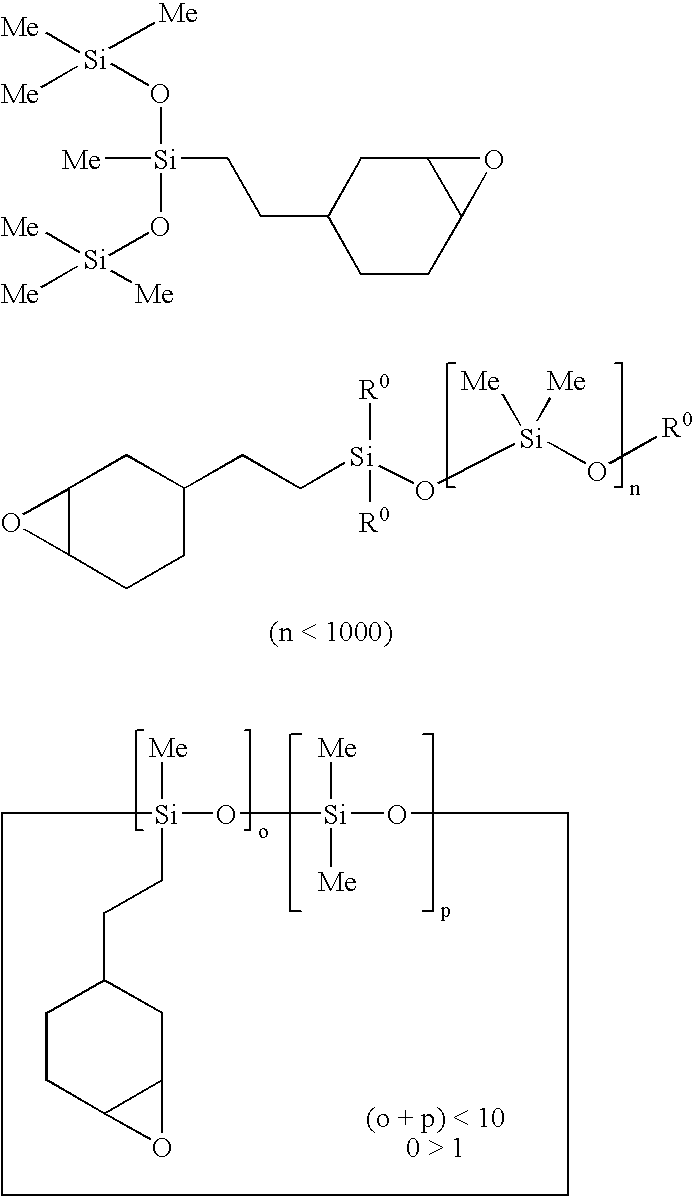
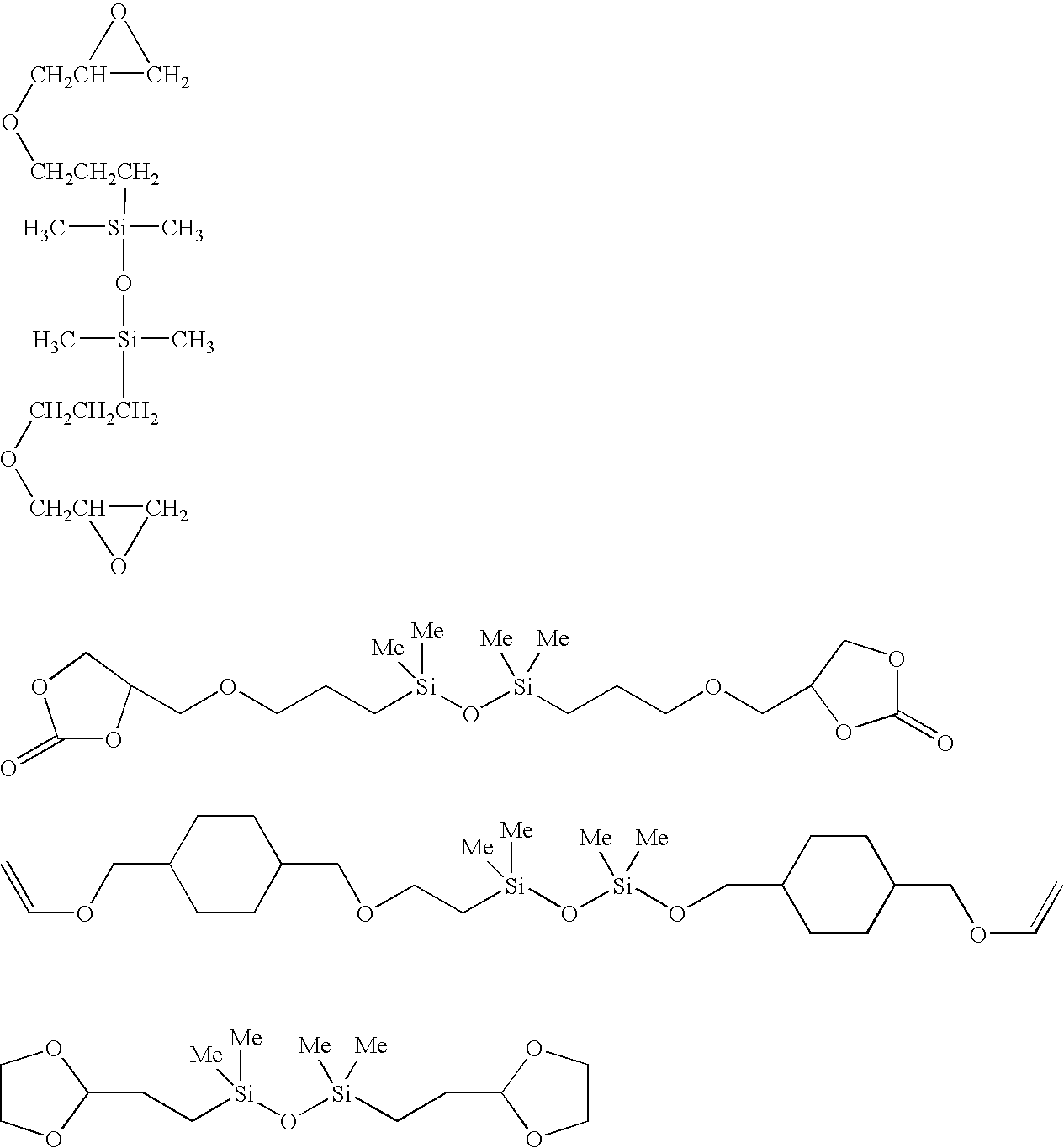
Cationically curable silicone compositions based on colloidal silica and anti-mist/anti-fouling hard coatings formed therefrom
a technology of colloidal silica and silicone composition, which is applied in the direction of instruments, optical elements, transportation and packaging, etc., can solve the problems of time needed for crosslinking, material is more easily subject to scratching and detrimental changes, and low hardness compared to glass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Flexform 40 Thermal Control Formulation
[0135] This solution is sold by Exxene for abrasion-resistant coatings.
[0136] This solution is applied by dipping to a polycarbonate sheet at 20° C. and is then dried at 25° C. for 10 min, followed by thermal crosslinking at 122° C. for 35 min.
[0137] The thickness of the film is two micrometers. A Taber abrasion resistance test is carried out according to standard T30-015 with a load of 500 g and 300 cycles with CS10-F abrasive wheels. A variation in gloss of 10% is found. % Haze=10%.
[0138] The pencil hardness of the coating ranges from 4 H to 6 H.
example 2
Preparation of a UV Formulation According to the Invention Without Anti-Mist Agent
[0139] 10 g of siloxane resin having a content of monomer (A) of greater than 90%, obtained by hydrosilylation of 4-vinylcyclohex-1-ene epoxide (VCMX), 1.25 g of photoinitiator systems including 20% of photoinitiator P1 dissolved in isopropanol, and 40 g of Highlink colloidal silica as a 30% solution in isopropanol are charged to a beaker.
[0140] The system is stable at ambient temperature for at least 6 months with the exclusion of light and heat. The solution is applied by dipping to a polycarbonate sheet.
[0141] The sheet is allowed to drain for one minute.
[0142] The system is crosslinked by passing, at the rate of 5 m / min, over a UV bench equipped with two 160 W / cm Hg lamps. The system is dry and very hard at the outlet of the bench.
[0143] The thickness of the film is 3 micrometers.
[0144] The pencil hardness is 3 H immediately and greater than 4 H after 24 hours.
[0145] An annealing at 150° C. ...
example 3
Preparation of a UV Formulation According to the Invention with Anti-Mist Agent
[0147] 10 g of siloxane resin having a content of monomer (A) of greater than 90%, 1.25 g of photoinitiator system including 20% of photoinitiator P1 dissolved in isopropanol, 40 g of Highlink colloidal silica in solution in isopropanol, and 0.5 g of silicone polyether Rhodorsil Oil 10646 are charged to a beaker.
[0148] The system is stable at ambient temperature for at least 6 months with the exclusion of light and heat. The solution is applied by dipping to a polycarbonate sheet.
[0149] The sheet is allowed to drain for one minute.
[0150] The system is crosslinked by passing, at the rate of 5 m / min, over a UV bench equipped with two 160 W / cm Hg lamps. The system is dry and very hard at the outlet of the bench.
[0151] The thickness of the film is 3 micrometers.
[0152] The pencil hardness is 3 H immediately and greater than 4 H after 24 hours.
[0153] The same Taber abrasion test is used. A variation in g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| mean diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com