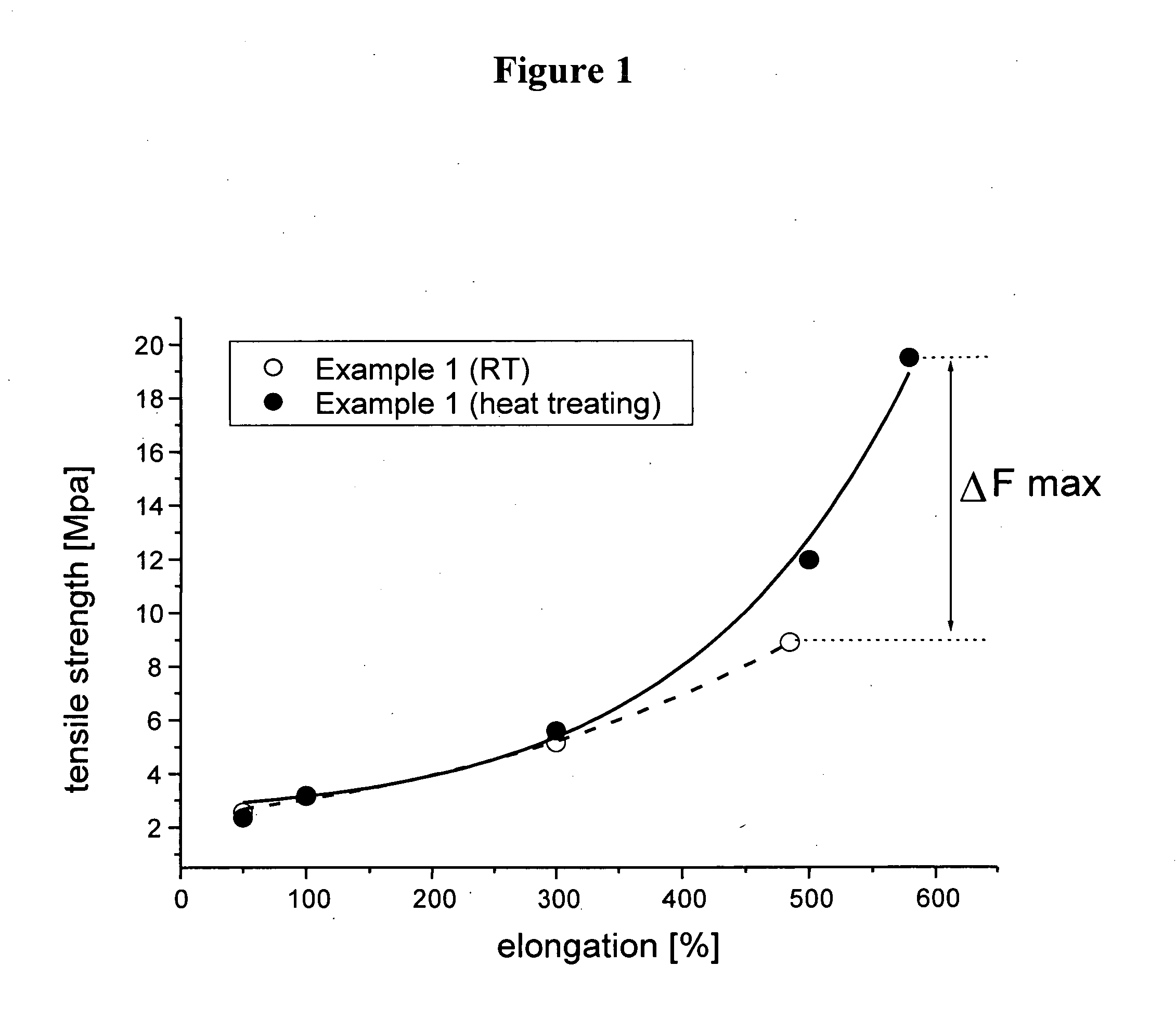
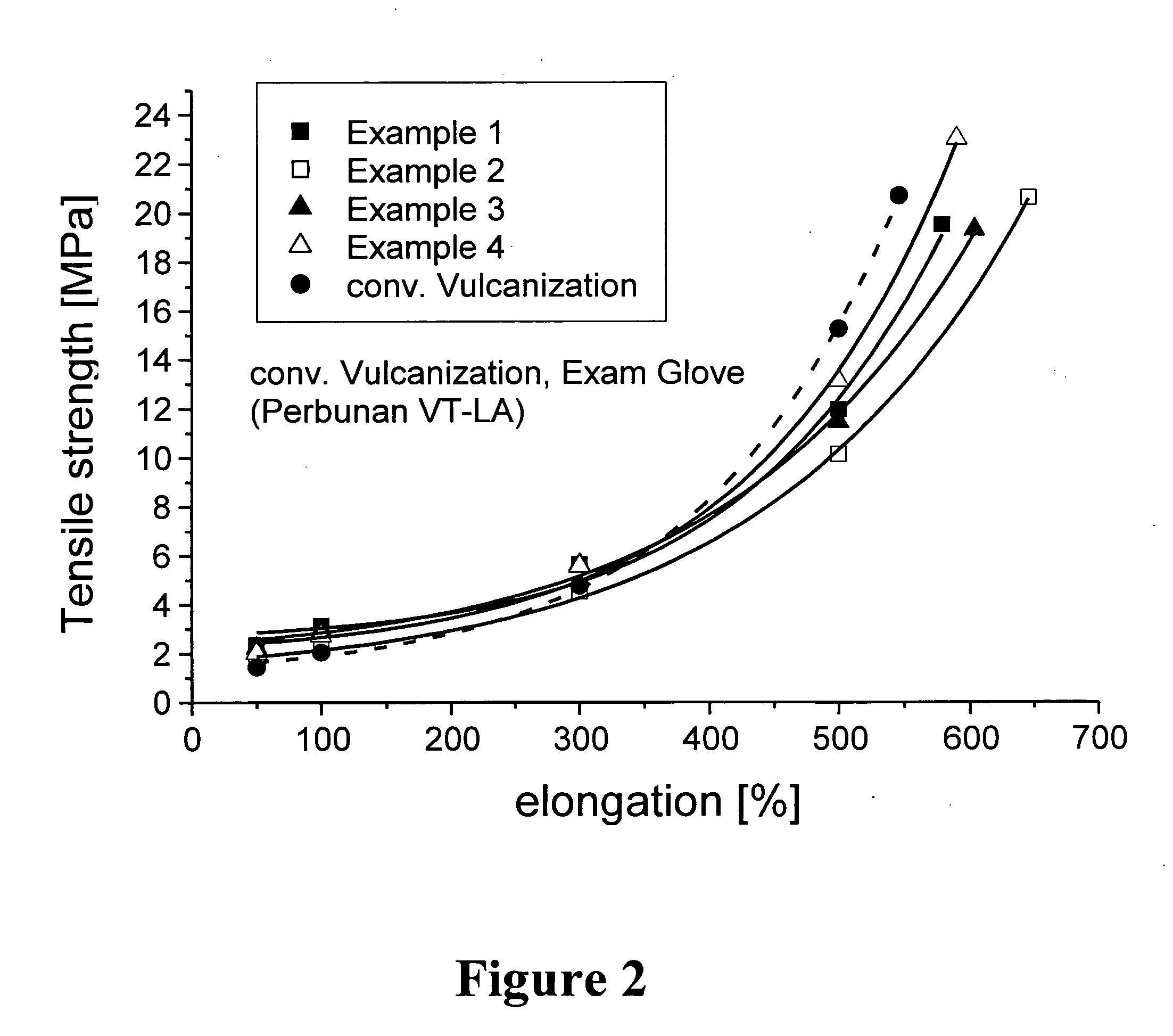
Polymer latex suitable for the preparation of dip-molded articles
a technology of polymer latex and dip-molded articles, which is applied in the field of polymer latex, can solve the problems of health risks, complicated process for making the latex compound, and health risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0106] 55 g of a 31% seed latex (particle size 36 nm) are heated to 40° C. in a nitrogen-purged autoclave with 750 g of water, 2 g Na dodecyl benzene sulfonate, 0.5 g of Na4EDTA, 0.05 g of Na formaldehyde sulfoxylate, 0.8 g of t-butyl hydroperoxide and an increment of the hard phase monomers mixture consisting of 94.3 g methylmethacrylate and 4.0 g methacrylic acid was added. After 1 h of polymerization an increment of following soft phase monomer / chain transfer agent mixture consisting of 270 g acrylo nitrile, 36 g methacrylic acid, 579 g butadiene, and 9 g t-dodecylmercaptan was added. Over a period of 7 hours an emulsifier / co-activator feed of 22.5 g Na dodecyl benzene sulfonate, 0.7 g Na formaldehyde sulphoxylate, and 300 g water was added. After a total polymerization time of 12 hours the total solids content was 48.0% corresponding to a conversion of 98%. The polymerization was short stopped by addition of 20 g of a 5% aqueous solution of diethylhydroxylamine. The pH was adjus...
example 2
[0107] 55 g of a 31% seed latex (particle size 36 nm) are heated to 40° C. in a nitrogen-purged autoclave with 750 g of water, 2 g Na dodecyl benzene sulfonate, 0.5 g of Na4EDTA, 0.05 g of Na formaldehyde sulphoxylate, 0.8 g of t-butyl hydroperoxide. For the hard phase a feed consisting of 66 g styrene, 28 g acrylonitrile and 4.0 g methacrylic acid was added within 1 hour. After 2 h of polymerization a feed of following soft phase monomer / chain transfer agent mixture consisting of 270 g acrylonitrile, 36 g methacrylic acid, 579 g butadiene, and 9 g t-dodecylmercaptan was added within a period of 5 hours. Parallel to the soft phase monomer feed over a period of 10 hours an emulsifier / co-activator feed of 22.5 g Na dodecyl benzene sulfonate, 0.7 g Na formaldehyde sulfoxylate, and 300 g water was added. After a total polymerization time of 15 hours the total solids content was 48.2% corresponding to a conversion of 98%. The polymerization was short stopped by addition of 20 g of a 5% a...
example 3
[0108] The polymerization was carried out like in Example 2 but for the hard phase a feed consisting of 80 g methylmethacrylate, 14 g butylacrylate and 4.0 g methacrylic acid was added within 1 hour.
[0109] After a total polymerization time of 15 hours the total solids content was 47.6% corresponding to a conversion of 97%. The polymerization was short stopped by addition of 20 g of a 5% aqueous solution of diethylhydroxylamine. The pH was adjusted by ammonia to pH 7.5 and the residual monomers were removed by vacuum distillation at 60° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tg | aaaaa | aaaaa |
| Tg | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com