Tumor-inhibiting protein and the use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of Full-Length cDNA for HCRP1 Gene
[0083] According to the positional candidate cloning strategy and human genomic sequence database, primers P1 (SEQ ID NO: 3) and P2 (SEQ ID NO: 4) were designed to amplify the HCRP1 cDNA from the human liver cDNA library (GIBCO BRL). The polymerase chain reaction (PCR) was performed with pre-denaturing for 2 min and 35 cycles of 94° C. for 30 s, 50° C. for 30 s, and 72° C. for 2 min. The PCR product was purified by using low melting agarose gel (see Sambrook, J., Fritsh, E. F., and Maniais, T., Molecular Cloning, Cold Spring Harbor Laboratory Press, 1989). Sequencing of the full-length cDNA was conducted by Bioasia, Co, Ltd (Shanghai, China).
[0084] The obtained 1917 bp full-length cDNA (SEQ ID NO:1) comprises a whole protein-encoding region (from 151 bp to 1341 bp), which codes for a protein consisting of 397 amino acids (SEQ ID NO:2).
example 2
RNA Blot Analysis of HCRP1 Expression in Multiple Human Tissues
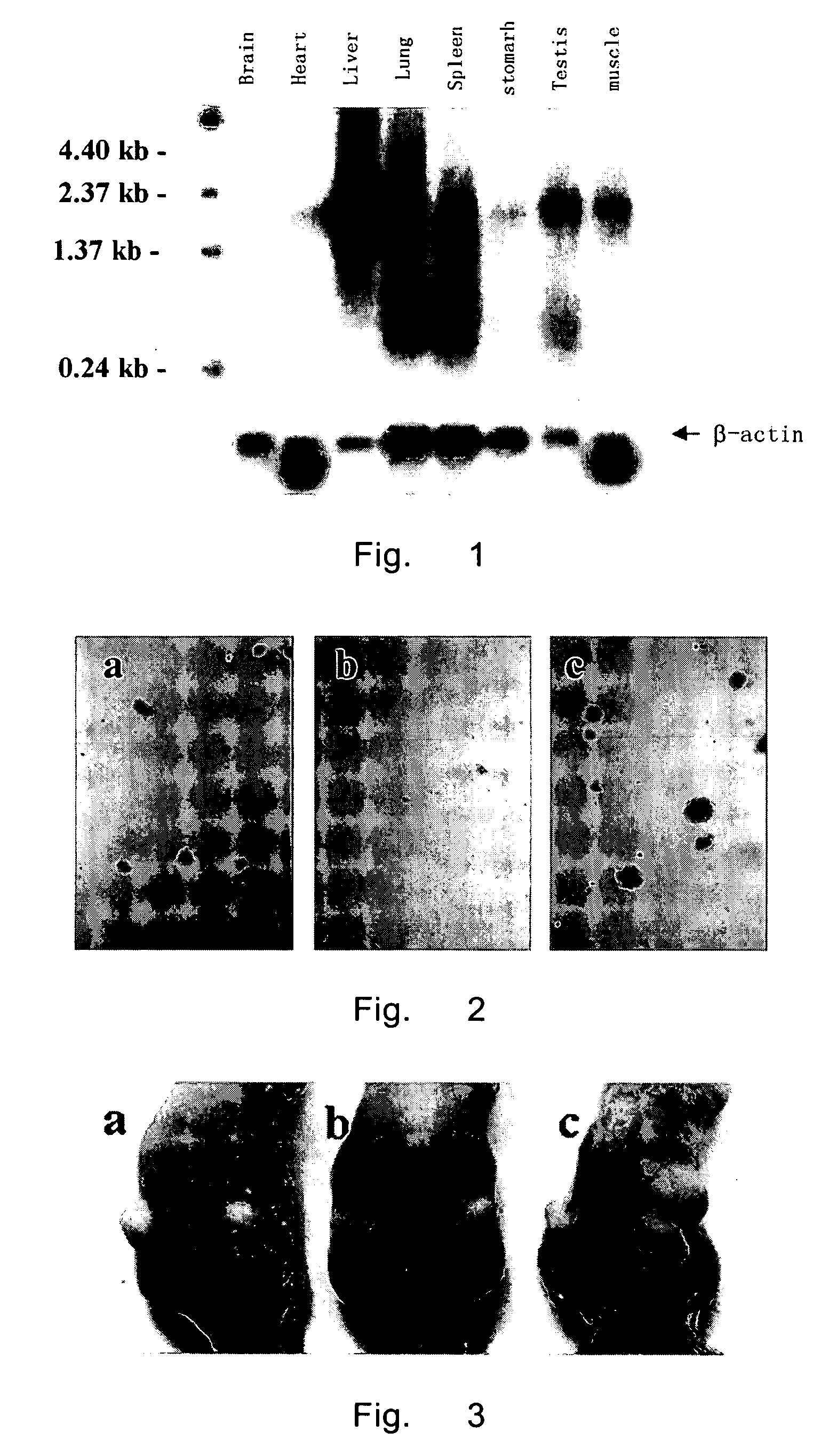
[0085] Multiple-tissue RNA blot membrane fixed with 8 different normal human tissues was placed in to a hybridization tube. 5 mil pre-hybridization solution (Kingrace) was added and the mixture was pre-hybridized at 65° C. for 30 minutes. Then the denatured probe of HCRP1 was added, which was randomly labeled with 32P(Random Primer DNA Labeling Kit, Takara), and hybridized overnight at 65° C. The blot membrane was washed with resin buffer I (0.3M NaCl, 0.03M citrate sodium (PH7.0), 0.05% sodium dodecyl sulfate) at room temperature; and then washed twice (20 min each) at 50° C. with resin buffer II (15 mM NaCl, 1.5M citrate sodium (PH7.0), 0.1% sodium dodecyl sulfate (SDS)). After that, the hybridized film was exposed to X-ray and radioautographed at −70° C.
[0086] As shown in FIG. 1, HCRP1 gene was highly expressed in liver, moderately in lung, spleen, testis and muscle, yet minimally or not expressed in brain, heart an...
example 3
Construction of Eukaryotic Expression Vector of HCRP1 Gene and Expression and Measurement of HCRP1 Protein
[0087] Primer P3 (SEQ ID NO:5) and primer P4 (SEQ ID NO:6) were designed according to the cDNA sequence of HCRP1 with the restriction endonuclease site of XbaI and EcoRI added to 5′ site of the primers, respectively. PCR reaction was performed by using the verified sequence of HCRP1 as template. The PCR product was recovered from the low melting agarose gel and then was digested for an hour with restriction enzyme XbaI and EcoRI (Takara, Co, Ltd.), followed by purification again. The vector pCMV-Tag2A (Stratagene) was also digested for an hour with the same restriction enzyme XbaI and EcoRI, followed by purification on low melting agarose gel. The two recovered products were ligated with T4 DNA ligase (Takara) at 16° C. overnight and then were introduced into E. Coli DH5a. The recombinant HCRP1 plasmid was obtained by screening and designated as pcDNA3-Flag-HCRP1. Primer P5 (SE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com