Methods for preparing catalysts supported on carbon nanotube networks
a carbon nanotube and network technology, applied in the preparation of amino compounds, physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, etc., can solve the problem of contaminating the product, difficult to determine the exact chemical nature of the active catalyst component within the reaction zone, and cost of separation from the reaction mixtur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Carbon Nanotube Network
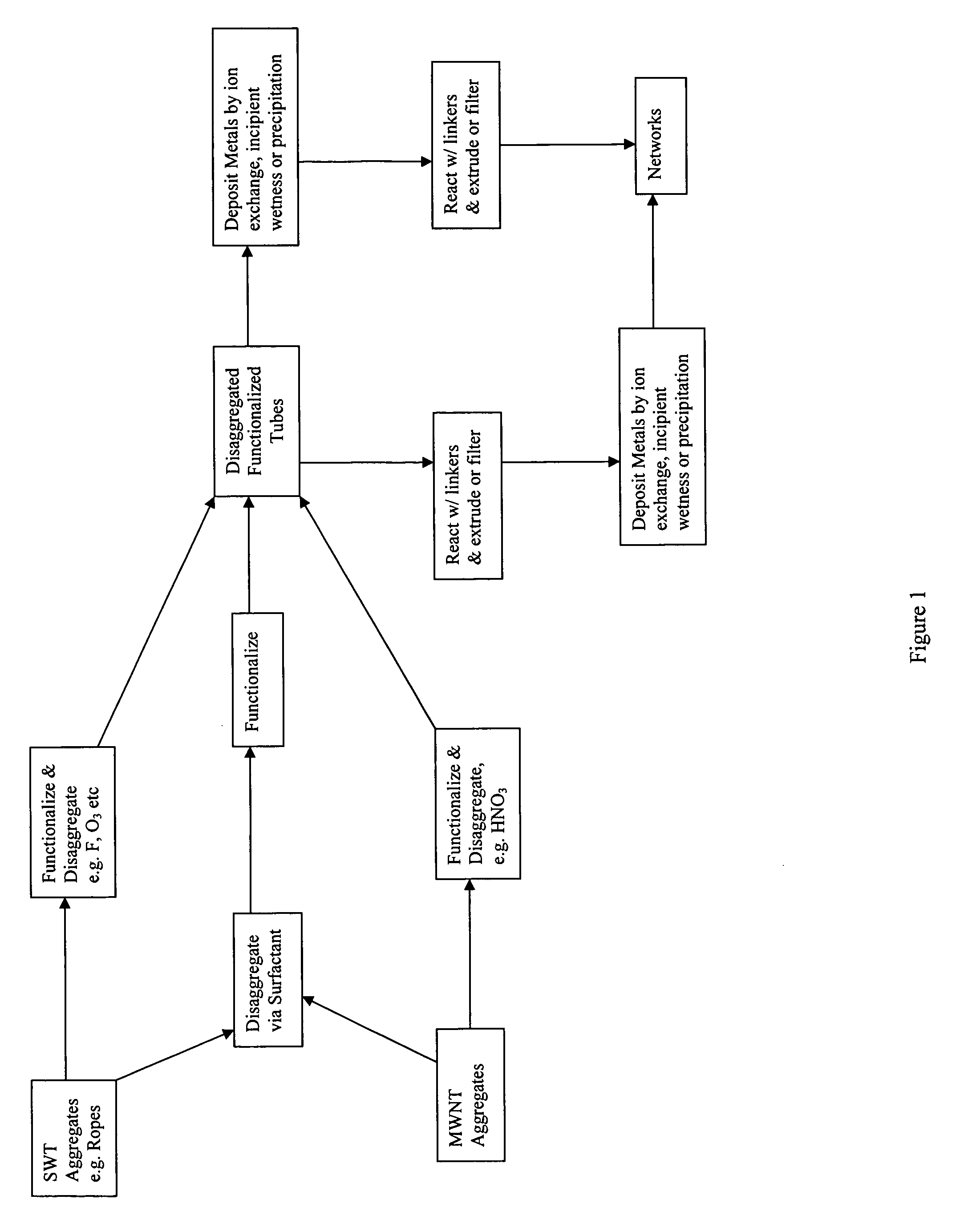
[0082] A covalently linked, carbon nanotube network is prepared by coupling a plurality of nanotubes together with molecules of a polyfunctional linker. The linker can have two or more reactive groups that are either the same or different such that at least one functional group on a linker molecule will react with one nanotube and at least a second functional group on the same linker molecule can react with a second nanotube thereby covalently linking the two nanotubes together. The functional groups on the polyfunctional linker can be the same or different and can be selected to react directly with an unfunctionalized nanotubes or selected to react with functional groups already present on the nanotubes.
[0083] Carbon nanotubes with carboxyl functional groups are linked using a diamine linker. Carbon nanotubes are slurried in 6M nitric acid in a two-necked, round bottom flask. The flask is fitted with a condenser with a water jacket in one neck and an overhe...
example 2
Carbon Nanotube Network Supported Catalyst via Post-Network Deposition
[0086] Functionalized carbon nanotubes contain a variety of diverse functional groups, i.e. anionic (e.g. —SO3H, —COOH), cationic (e.g. —N(R1, R2, R3)+ or more or less complex organic groups like amino, amide, ester, nitrile, epoxy or other reactive centers. Preparation of a metal loaded carbon nanotube composite can then be carried out by the preparation of functionalized carbon nanotubes, metallation either by ion-exchange or impregnation with a metal compound and the reduction of metal compound to metallic state.
[0087] 30 ml 0.25 wt % PdCl2 / HCl solution is loaded in a flask with 20 ml water. The pH of the solution at this point is around 4. 1.001 g of CNT mat containing carbon nanotube network made in Example 1 are added to the solution. The slurry is stirred at room temperature for 24 hours. The filtration of the slurry yielded a light yellow filtrate, indicating that not all of the Pd ions are loaded on the...
example 3
Carbon Nanotube Network Supported Catalyst via Pre-Network Deposition
[0089] Metal catalyst can also be pre-deposited on functionalized carbon nanotubes via ion-exchange or impregnation pathways. A Pd catalysts supported on carbon nanotubes is prepared by incipient wetness impregnation. First, 10 grams of CC-type carbon nanotubes are placed in a 250-cc round bottom flask and oxidized by 63% nitric acid under reflux condition for four hours. After thorough washing with de-ionized water, the oxidized nanotubes are impregnated with Pd(NO3)2 / acetone solution to yield a metal loading of 5%.
[0090] Pd-loaded nanotubes are then activated to an N-hydroxysuccinimide (NHS) ester by carbodiimide coupling using 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide. The product is then washed with dioxane and methanol then dried under vacuum to yield NHS ester-activated nanotubes.
[0091] NHS ester-activated nanotubes are cross-linked by the diamine, ethylenediamine, by adding ethylenediamine in 0.2M NaH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface areas | aaaaa | aaaaa |
| pore diameters | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com