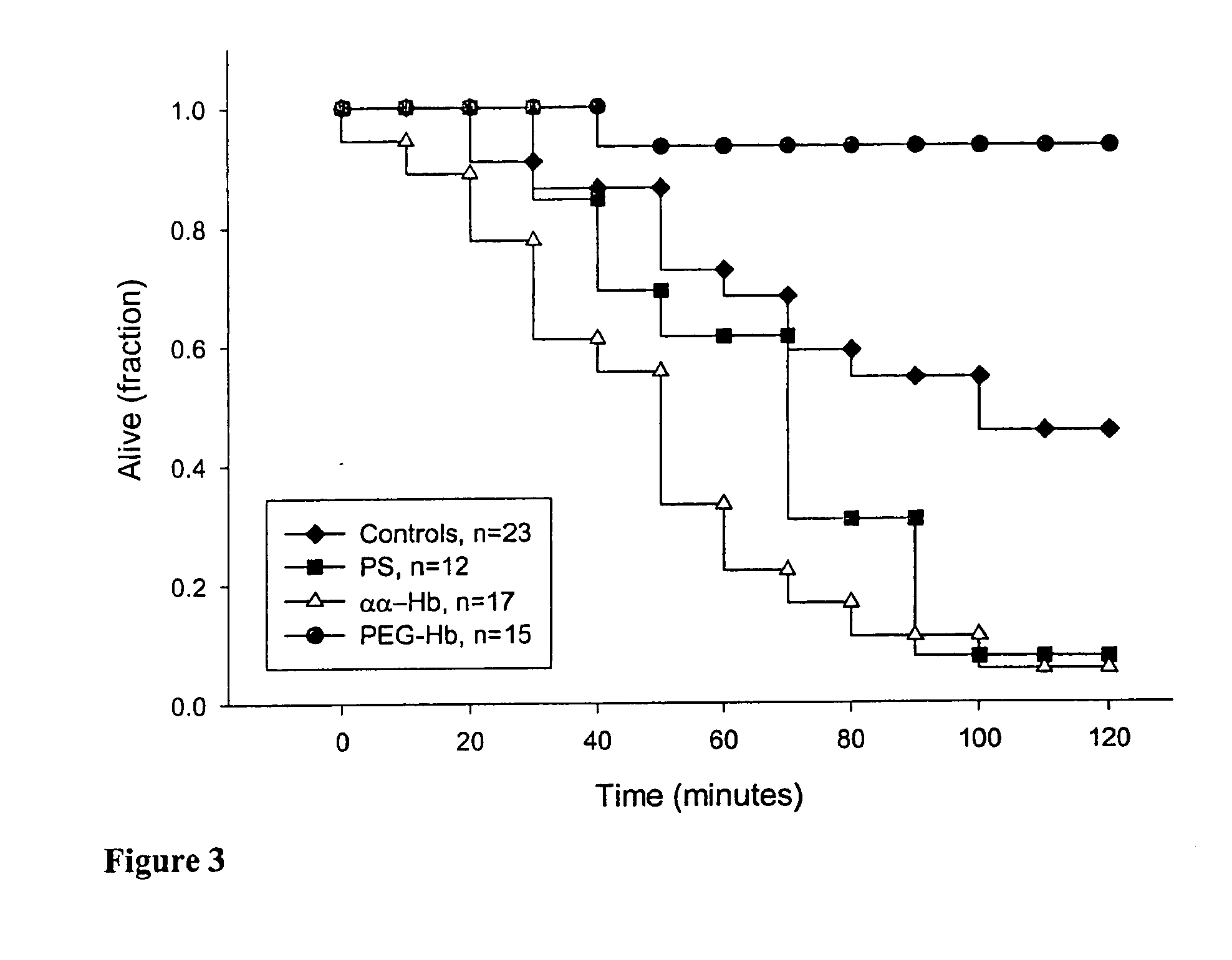
Methods to enhance hemodynamic stability using oxygen carrying compositions
a technology of hemodynamic stability and oxygen carrying composition, which is applied in the direction of drug compositions, peptide/protein ingredients, extracellular fluid disorder, etc., can solve the problems of widespread use of whole blood nor hbocs for prophylactic indications, unsuitable prophylactic use of human blood, and somewhat questionable practice, so as to enhance hemodynamic stability, monitor the hemodynamic stability of the patient, and enhance hemodynamic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Stroma-Free Hemoglobin
Step 1: Procurement of Outdated Red Blood Cells
[0084] Outdated packed red blood cells are procured from a commercial source, such as the San Diego Blood Bank or the American Red Cross. Preferably, outdated material is received not more than 45 days from the time of collection. Packed RBCs (pRBCs) are stored at 4±2° C. until further processed (1-7 days). All units are screened for viral infection and subjected to nucleic acid testing prior to use.
Step 2: Pooling of Outdated Blood
[0085] Packed red blood cells are pooled into a sterile vessel in a clean facility. Packed red blood cell volume is noted, and hemoglobin concentration is determined using a commercially available co-oximeter or other art-recognized method.
Step 3: Leukodepletion
[0086] Leukodepletion (i.e. removal of white blood cells) is carried out using membrane filtration. Initial and final leukocyte counts are made to monitor the efficiency of this process.
Step 4: Cell Separa...
example 2
Modification of Stroma Free Hemoglobin
Step 1: Thiolation
[0093] Thiolation is carried out using 10-fold molar excess iminothiolane over hemoglobin for 4 hours at 4±2° C. with continuous stirring.
[0094] Reaction conditions:
[0095] 1 mM hemoglobin (tetramer) in RL (pH 7.0-7.5) or PBS (pH 7.4)
[0096] 10 mM iminothiolane in RL (pH 7.0-7.5) or PBS (pH 7.4)
[0097] The ratio of 1:10 SFH:iminothiolane and reaction timing were optimized to maximize the number of PEGylated thiol groups and to minimize product heterogeneity.
Step 2: PEGylation of Thiolated Hemoglobin
[0098] Thiolated hemoglobin is PEGylated using a 20-fold molar excess of Mal PEG (with an alkyl or phenyl linker) based on starting tetrameric hemoglobin concentration. The hemoglobin is first allowed to equilibrate with the atmosphere to oxygenate the hemoglobin. The reaction takes place for 2 hours at 4±2° C. with continuous stirring.
[0099] Reaction conditions:
[0100] 1 m thiolated hemoglobin in RL or PBS (pH 7.4)
[0101] 20...
example 3
Physiochemical Analysis of MalPEG-Hb
Methodology for Physicochemical Analysis
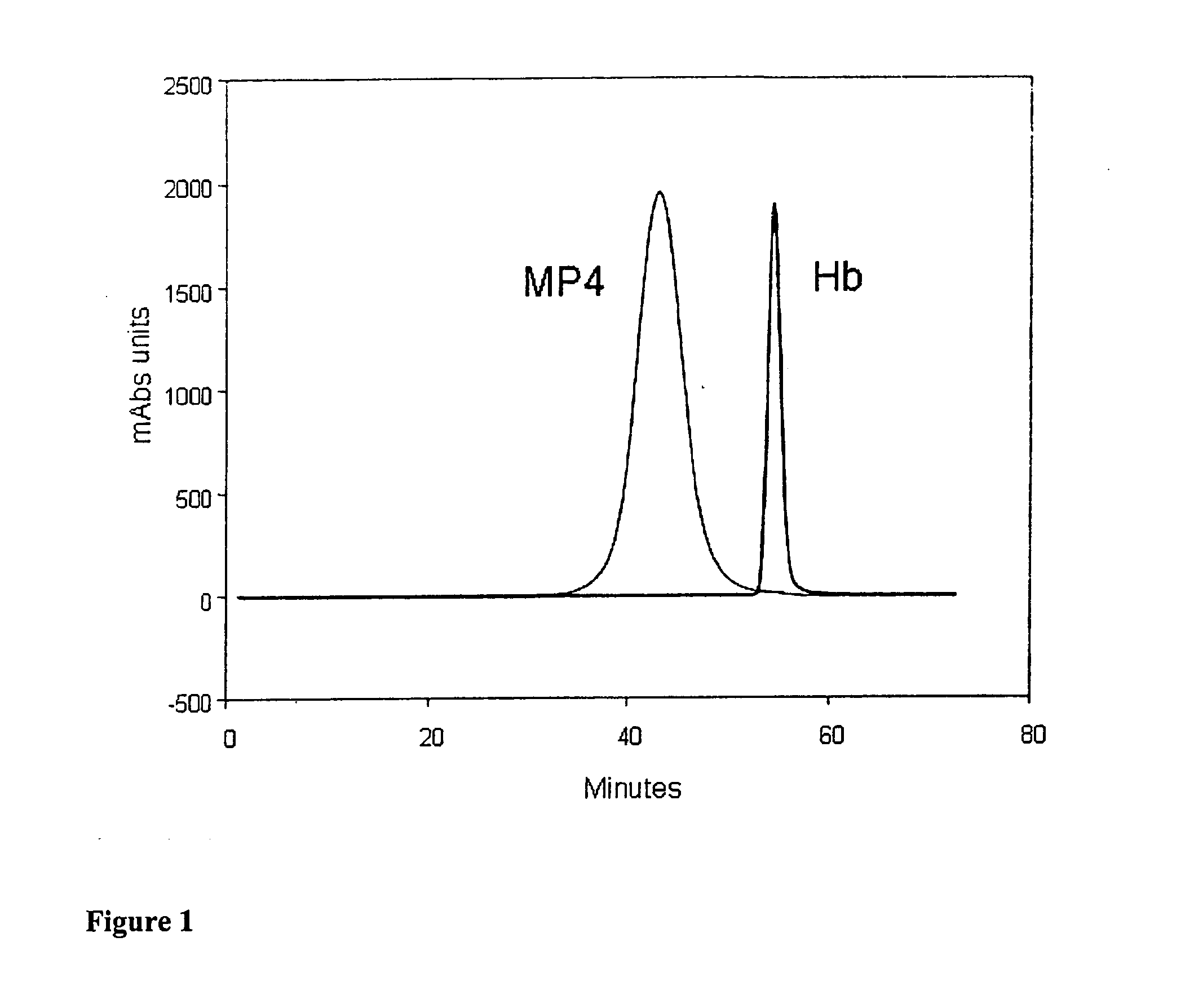
[0106] Homogeneity and molecular size of the MalPEG-Hb blood substitute are characterized by Liquid Chromatography (LC). Analytical LC is used to evaluate homogeneity of the PEGylated hemoglobin and extent of removal of unreacted Mal-PEG. Absorbance at 540 urn is used to evaluate hemoglobin and resolves PEGylated hemoglobin from unreacted hemoglobin by peak position. Absorbance at 280 nm is used to resolve PEGylated hemoglobin from free Mal-PEG, which absorbs in the ultraviolet (UV) spectrum due to the ring structures in MalPEG.
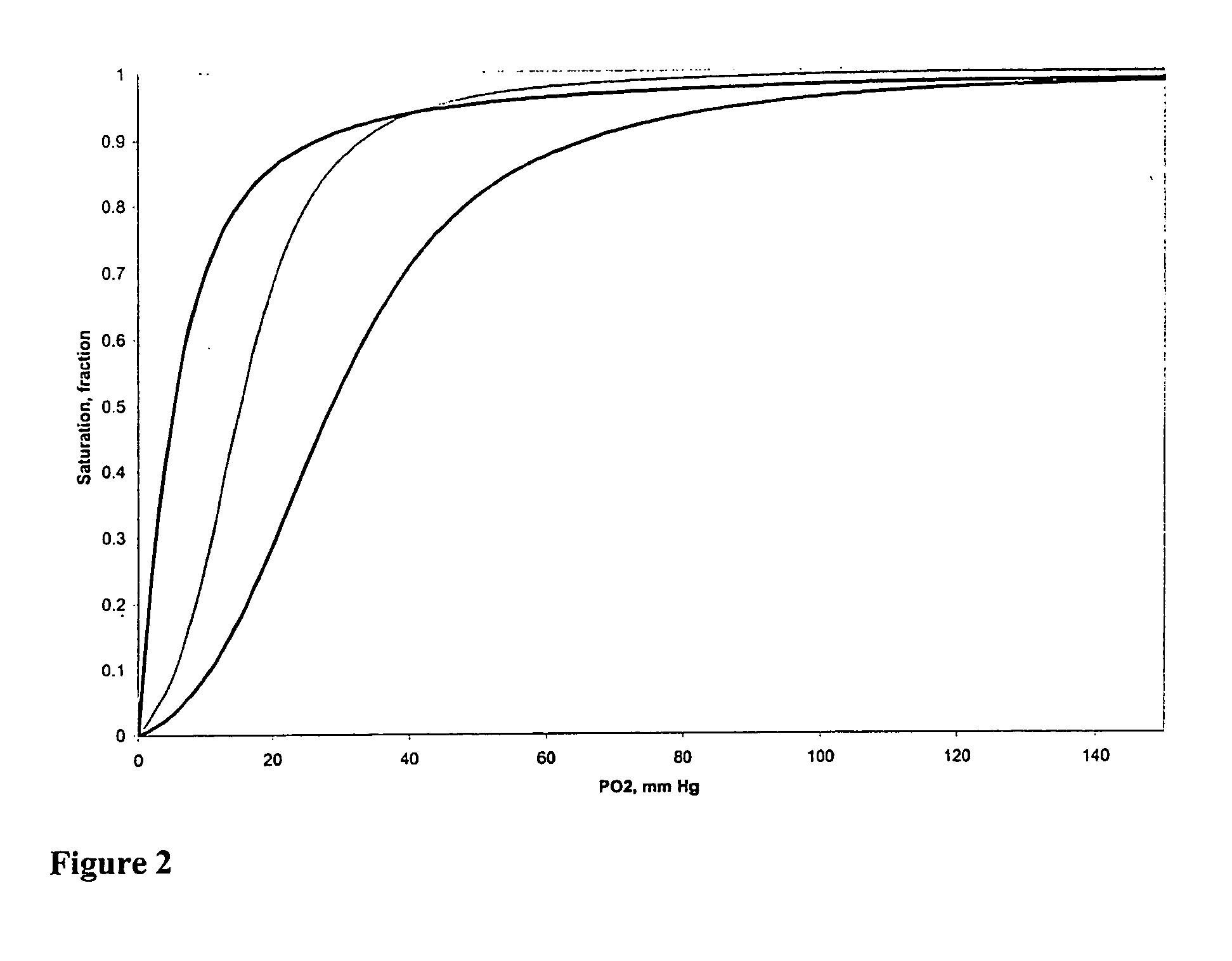
[0107] Optical spectra are collected using a rapid scanning diode array spectrophotometer (Milton Roy 2000 or Hewlett Packard Model 8453) in the Soret and visible regions for analysis of hemoglobin concentration and percent methemoglobin by multicomponent analysis (Vandegriff K. D., and R. E., Shrager. Evaluation of oxygen equilibrium binding to hemoglobin by rapid-scanning spcetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| systolic pressure | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com