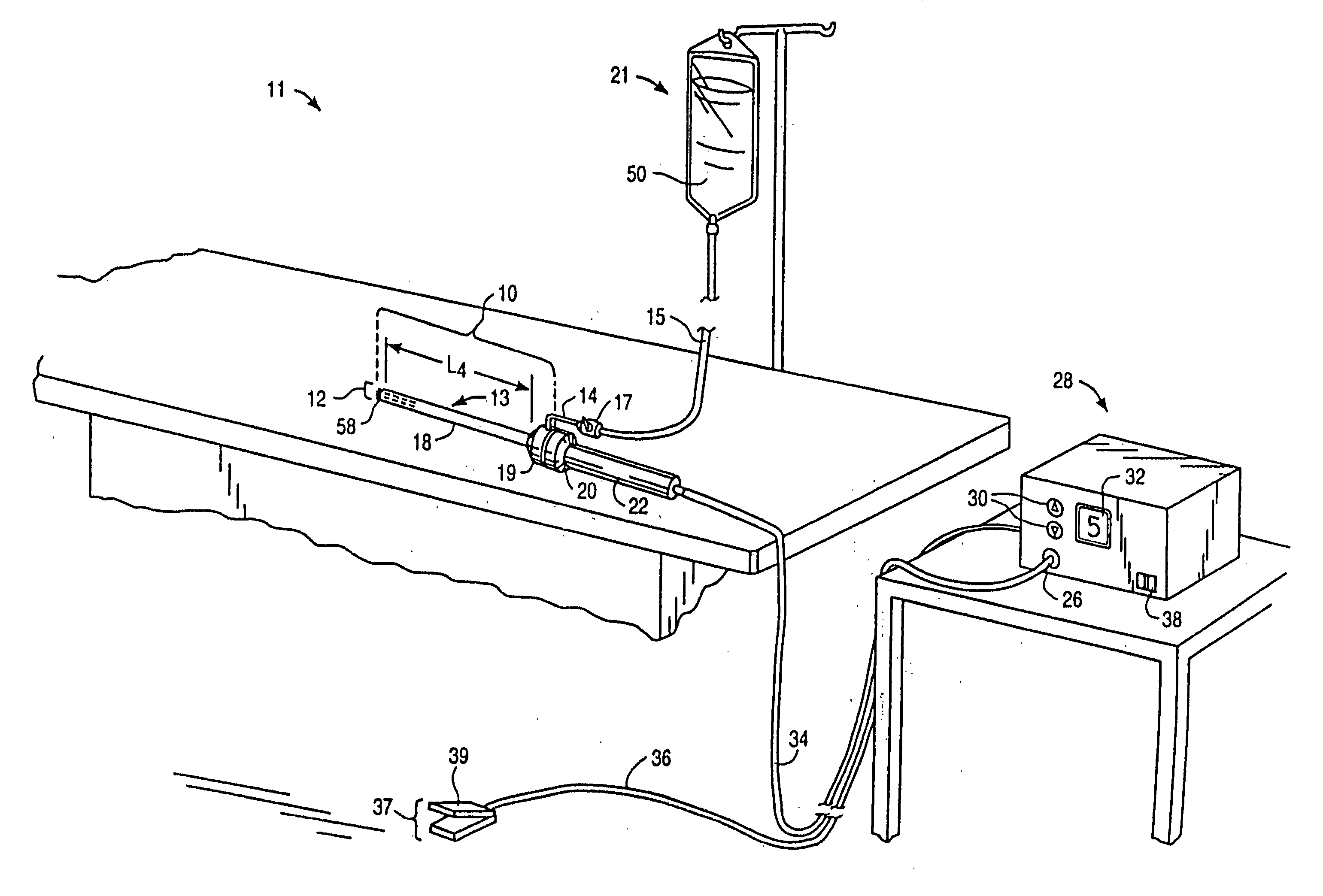
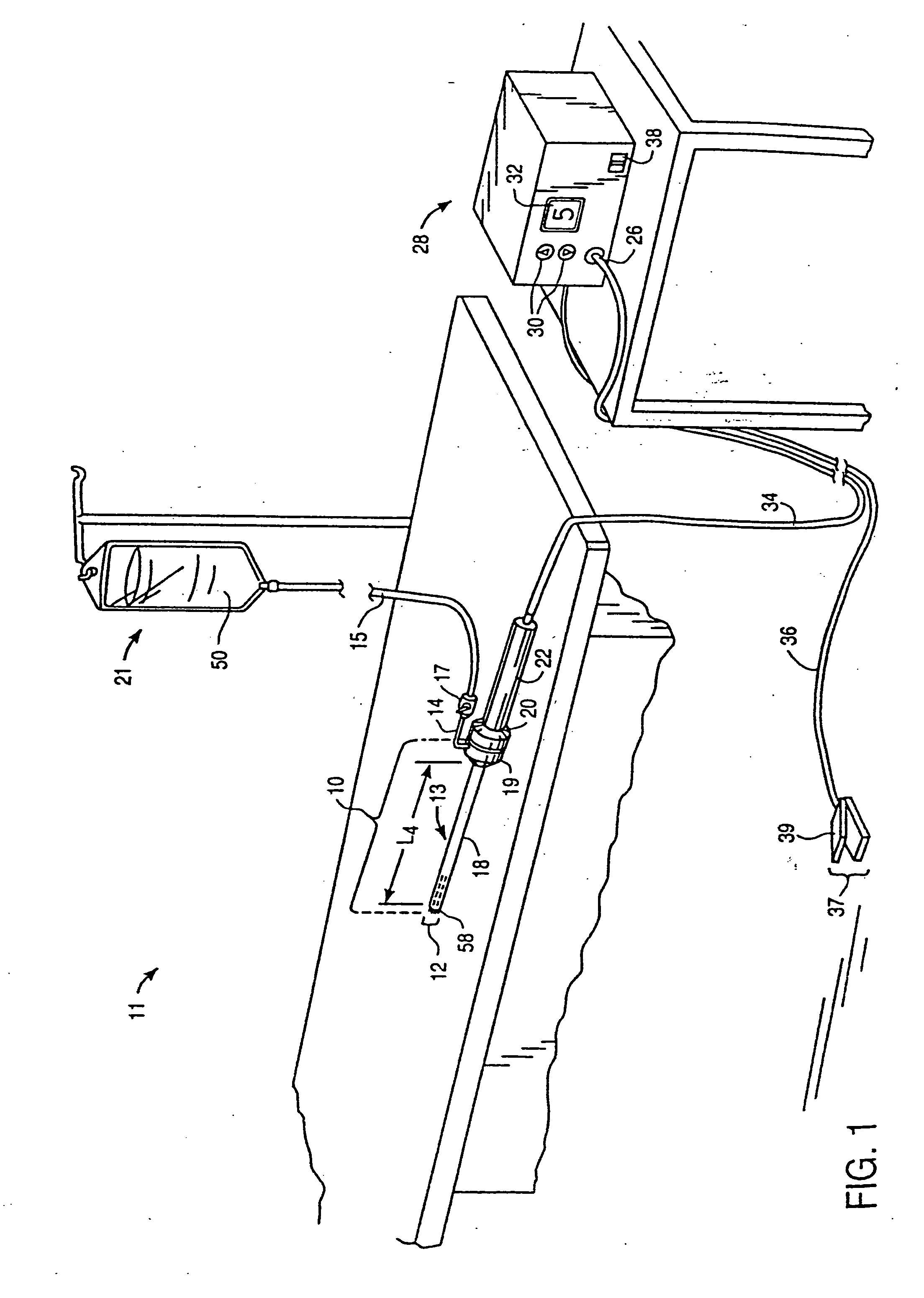
Systems and methods for electrosurgical treatment of fasciitis
a technology of electrosurgical treatment and fasciitis, applied in the field of electrosurgical treatment of fasciitis, can solve the problems of insufficient blood flow to the heart, patients are too sick to successfully undergo bypass surgery, previous endovascular and/or bypass surgery attempts have failed to provide adequate revascularization of the heart muscle, etc., to achieve sufficient rf energy, facilitate healing process, and vascularize the tendon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
second embodiment
[0118] In a second embodiment, the detection system can be an ultrasound guidance system that transmits sound waves onto the heart wall to facilitate canalization of the heart.
[0119] Referring to FIGS. 18 and 19, an ultrasound tissue thickness measuring system may be incorporated within an electrosurgical instrument of the invention, e.g., probe 100 or catheter 200, to measure the thickness of the heart wall 260 adjacent to active electrode 270, and thereby allow the surgeon to pre-set the depth of each channel using adjustable stop 352 on handpiece 340 (FIG. 11) before energizing catheter 200 / probe100 and ablating the heart tissue. In the embodiment shown in FIG. 18, an ultrasonic transducer 310, affixed to the distal end of the instrument, and connected to an external ultrasonic generator and sensing system (not shown) via lead 312, transmits pulses of ultrasound into the heart tissue in the form of emitted ultrasound signal 314, and the ultrasound generator and sensing system mea...
third embodiment
[0120] A third embodiment is shown in FIG. 19 wherein an ultrasonic transducer 310 is affixed to the distal end of electrosurgical catheter 200 / probe 100, and connected to an external ultrasonic generator and sensing system (not shown) via leads 312, and transmits pulses of ultrasound into the heart tissue in the form of emitted ultrasound signal 314. The ultrasound generator and sensing system measures the delay time for reflected ultrasound signal 316 to return from the boundary of the heart wall at the surface of epicardium 268 to the sensing system. This measured delay time can be translated into the distance between active electrode 270 and the surface of the epicardium 268. In this arrangement, the surgeon can observe where the channel 264 reaches the preferred distance from the epicardium 268, and can interrupt the application of power and advancement of catheter 200 / probe 100. In one embodiment, the preferred minimum thickness of the uncanalized heart wall 260 (i.e., the min...
fourth embodiment
[0121] A fourth embodiment is shown in FIG. 20, in which an electrosurgical instrument, e.g., probe 100 / 202 includes a small diameter tissue electrical impedance measurement sensor 319 which extends distally from active electrode(s) 270 by a distance L1. Impedance measurement sensor 319 detects the outer surface of the epicardium 268 as sensor 319 enters a region of different electrical impedance (viz, the fluid-filled cavity surrounding the heart). In the present embodiment, a sensor tip 320 may include a first impedance measurement electrode 321 and a second impedance measurement electrode 323. A small, high-frequency potential is applied between first and second impedance measurement electrodes 321 and 323 causing current flow between first and second impedance measurement electrodes 321 and 323 as indicated by current flux lines 322. As the first and second electrodes 321 and 323 emerge from the epicardium 268 into cavity 318 surrounding the heart, the change in electrical imped...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com