Medical devices with nanoporous layers and topcoats
a technology of nanoporous layers and medical devices, applied in the direction of medical preparations, plant/algae/fungi/lichens ingredients, prosthesis, etc., can solve the problems of porous alumina with porous alumina has severe mechanical integrity problems, and polymer coatings. , to achieve the effect of reducing the risk of delamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example b
2. EXAMPLE B
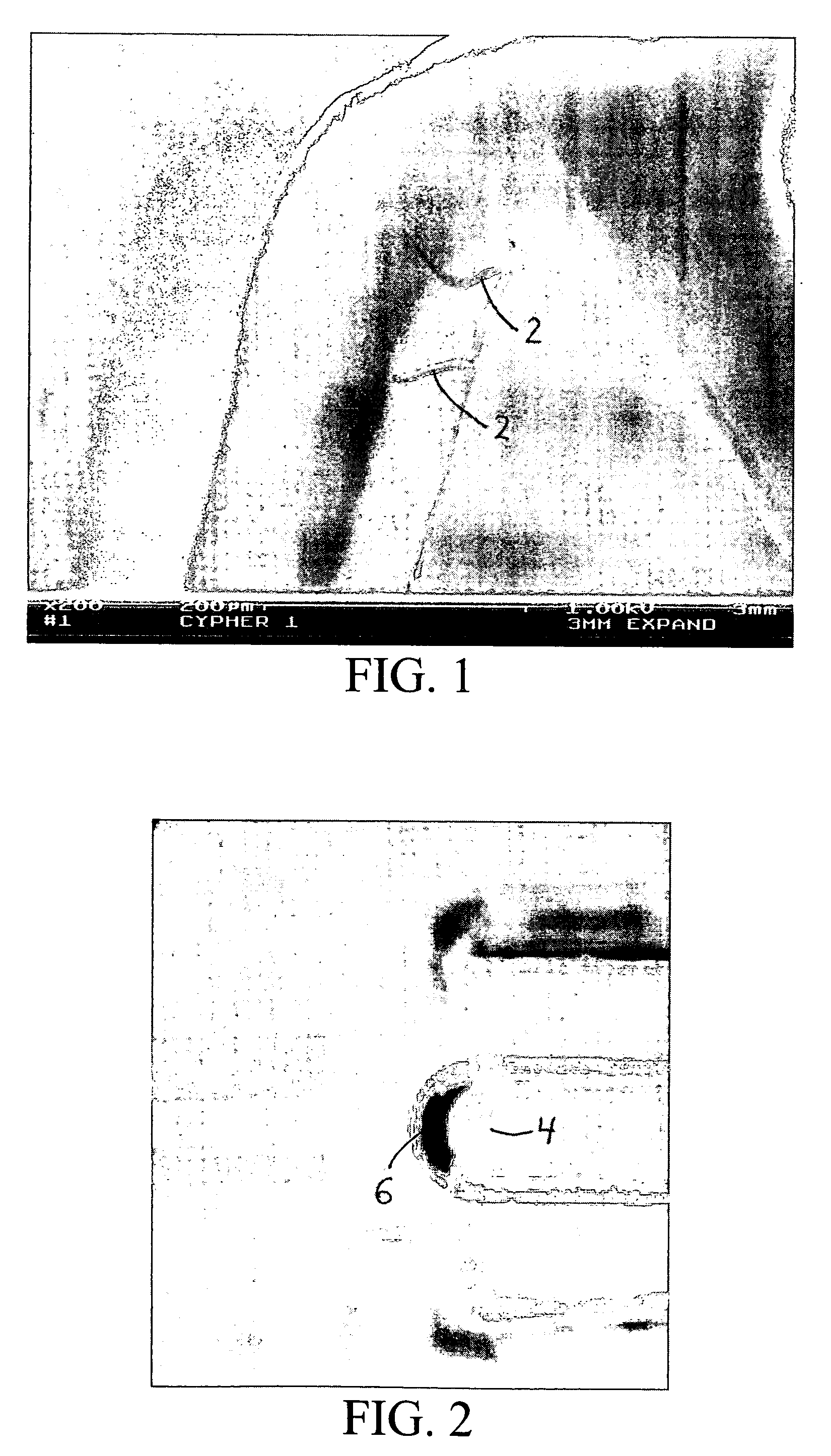
[0205] In another specific example, a coronary stent is co-sputtered with L605 (1.5 A / s) and magnesium (12 A / s) in 2×10-3 torr Argon for a resulting alloy coating that is approximately 80% by weight of magnesium. The stent is dealloyed using a 1% HNO3 at about 10 Celsius for about 5 minutes, followed by an anneal at about 600° Celsius for 10 minutes at about 10−5 torr vacuum with a ramp rate of about 200° Celsius / minute. This process produces a dealloyed layer as depicted in the scanning electron micrograph in FIG. 19. The resulting porous zone is approximately 5% by weight of magnesium and has a range of pore sizes from about 10 nm to about 200 nm. In a further embodiment, this stent is loaded with rapamycin using the same procedure as disclosed in Example A, resulting in an initial payload of about 85 micrograms. Place in an in vivo porcine coronary artery stent model results in a 7 day tissue concentration of about 0.80 ng / mg of tissue as measured by tandem MS / MS HPLC...
example c
3. EXAMPLE C
[0206] In still another specific example, a coronary stent undergoes a lower layer sputter deposition with L605 (1.5 A / s) and magnesium (12 A / s) in 2×10-3 torr Argon, and followed by an additional upper layer co-sputtering with L605 (3.1 A / s) and Mg (9.7 A / s) in 2×10-3 torr Argon, for a resulting alloy coating has a lower layer thickness of about 750 nm and an upper layer with a thickness of about 75 nm. Optionally, one or both sputtering steps may be repeated one or more times, in an alternating or other desired order, to create a layered columnar porous zone. In one embodiment, shown in FIG. 20, an additional two high magnesium content layers, with one lower magnesium content layer is sputtered to produce a five layer porous stent surface. The stent is dealloyed using a 1% HNO3 at about 10 Celsius for about 5 minutes, followed by an anneal at about 600° Celsius for 10 minutes at about 10−5 torr vacuum with a ramp rate of about 200° Celsius / minute. The resulting porous ...
example d
4. EXAMPLE D
[0208] In still example, a coronary stent undergoes a lower layer sputter deposition with L605 (3.1 A / s) and magnesium (9.7 A / s) in 2×10-3 torr Argon, for a resulting alloy coating with about 30% magnesium content by weight. The stent undergoes thermal dealloying by heating the porous zone with a heat source at about 600° Celsius for 10 minutes at about 10−5 torr vacuum with a ramp rate of about 200° Celsius / minute. The resulting porous zone is about 10-15% by weight of magnesium with a pore size range of about 1 nm to about 25 nm, but with occasional larger spaces up to about 500 nm or more, as depicted in FIGS. 21A and 21B. Although not wishing to be bound by the theory, it is hypothesized that the different macroscopic morphologies as illustrated in FIGS. 21A and 21B may result from different intrinsic film strains prior to the thermal dealloy process. In one further embodiment, the stent is loaded with rapamycin using an alternative loading procedure as described in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com