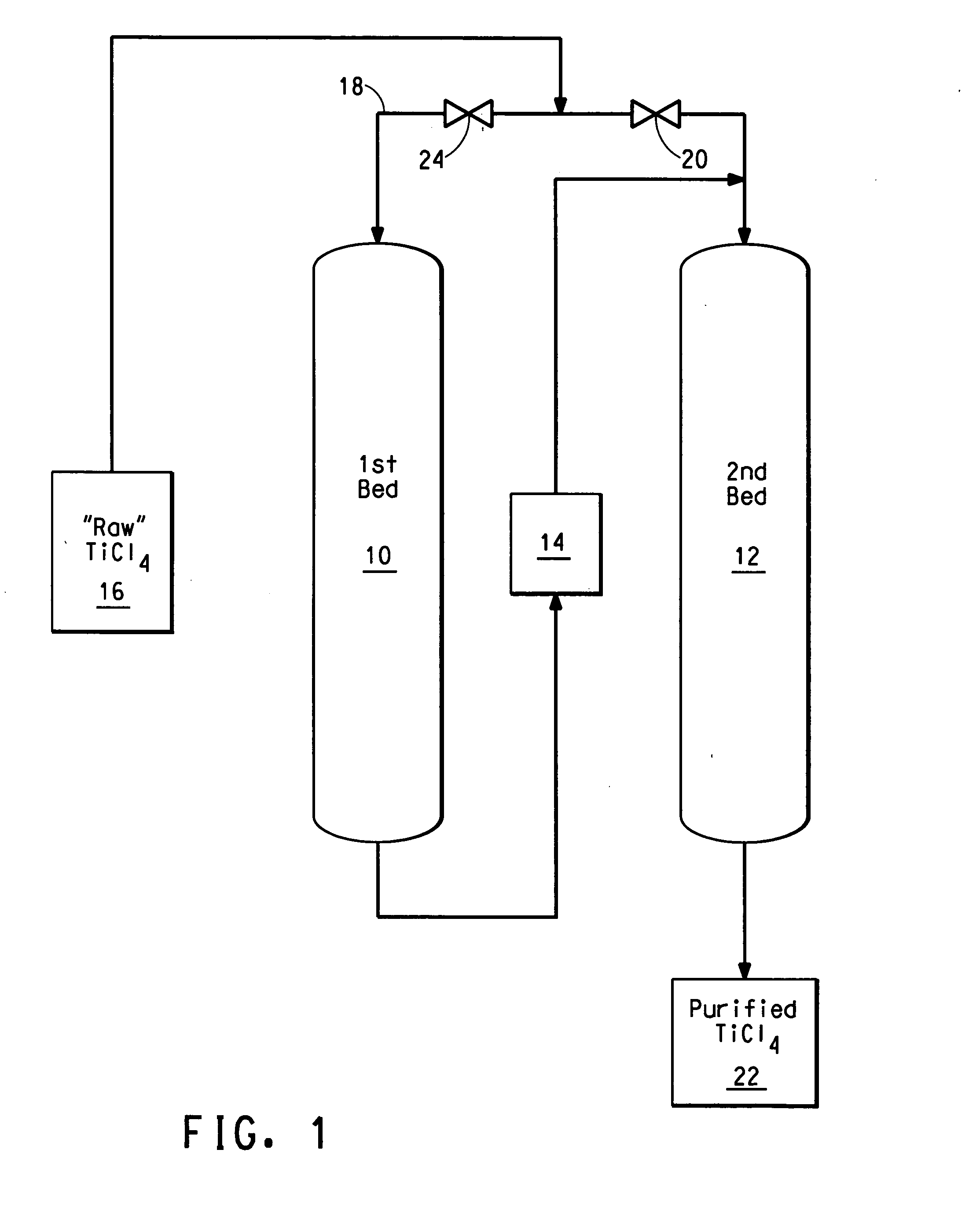
Process for purifying titanium chloride-containing feedstock
a technology of titanium chloride and feedstock, which is applied in the direction of arsenic compounds, other chemical processes, separation processes, etc., can solve the problems of inability to meet the requirements of commercial processes or a full scale production unit, no known direct methods, and time-consuming analytical methods that can take several hours to complete each. to achieve the effect of running the purification process more efficiently
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
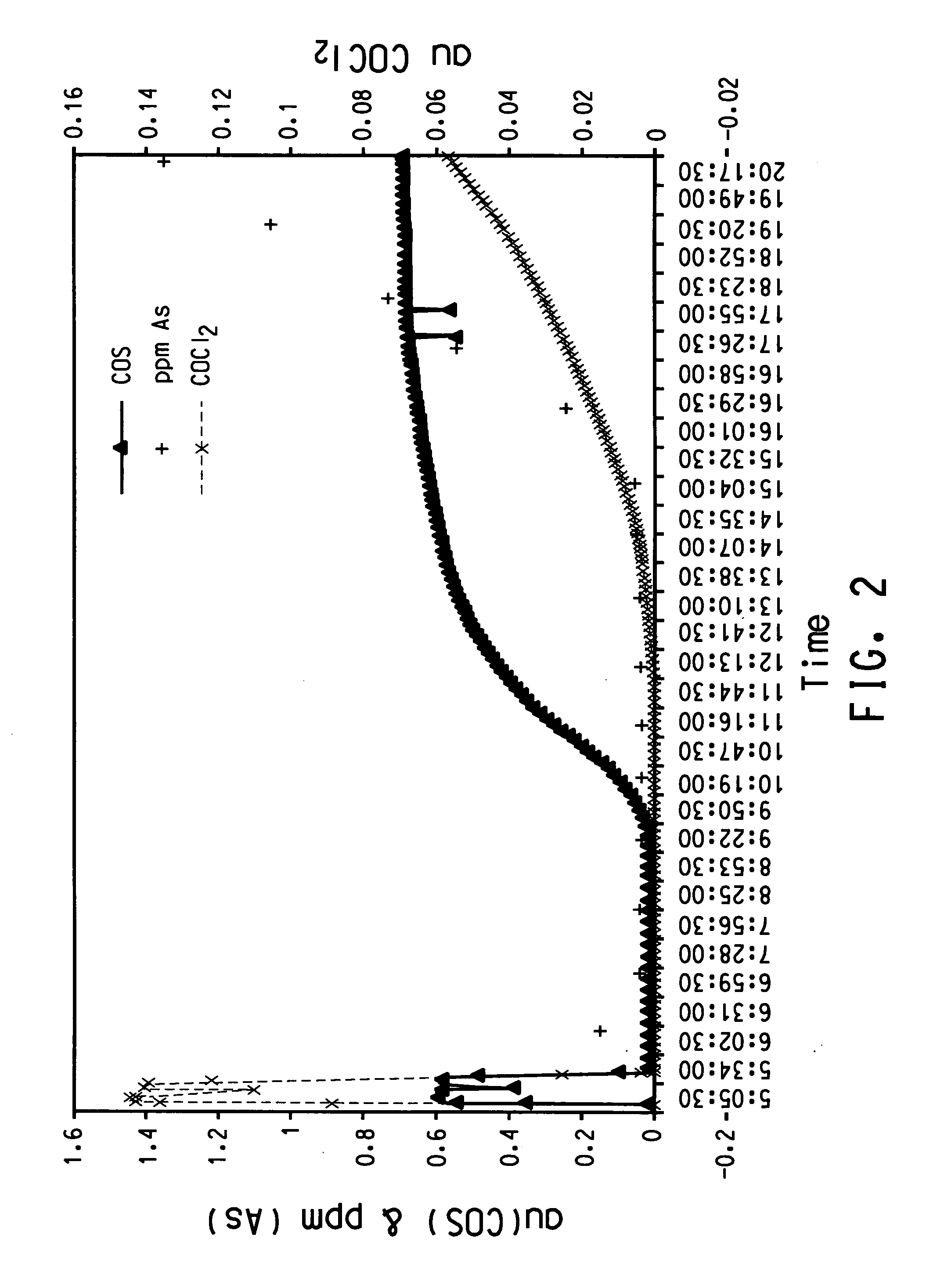
[0046] In this example, the tracker species concentration in the product was measured on a continuous basis using an in-line analyzer. A sample of anhydrous titanium tetrachloride containing 5 ppm arsenic on a titanium tetrachloride basis was used in this Example 1. The feed was also measured by FTIR to contain 0.588 absorbance units (au) of COS and 0.144 au of COCl2. The titanium tetrachloride was introduced into the top of the column and allowed to flow by gravity through the column. The titanium tetrachloride collected at the bottom of the column was passed through an FTIR analyzer that analyzed for the presence of the following two tracker species: phosgene and carbonyl sulfide.
[0047]FIG. 2 is a plot of absorbance units for each of the two tracker species that shows how the tracker species pattern the concentration of arsenic in the product. FIG. 2 also shows the concentration of tracker species in the feedstock when the feed was passed through the FTIR before the start of the ...
example 2
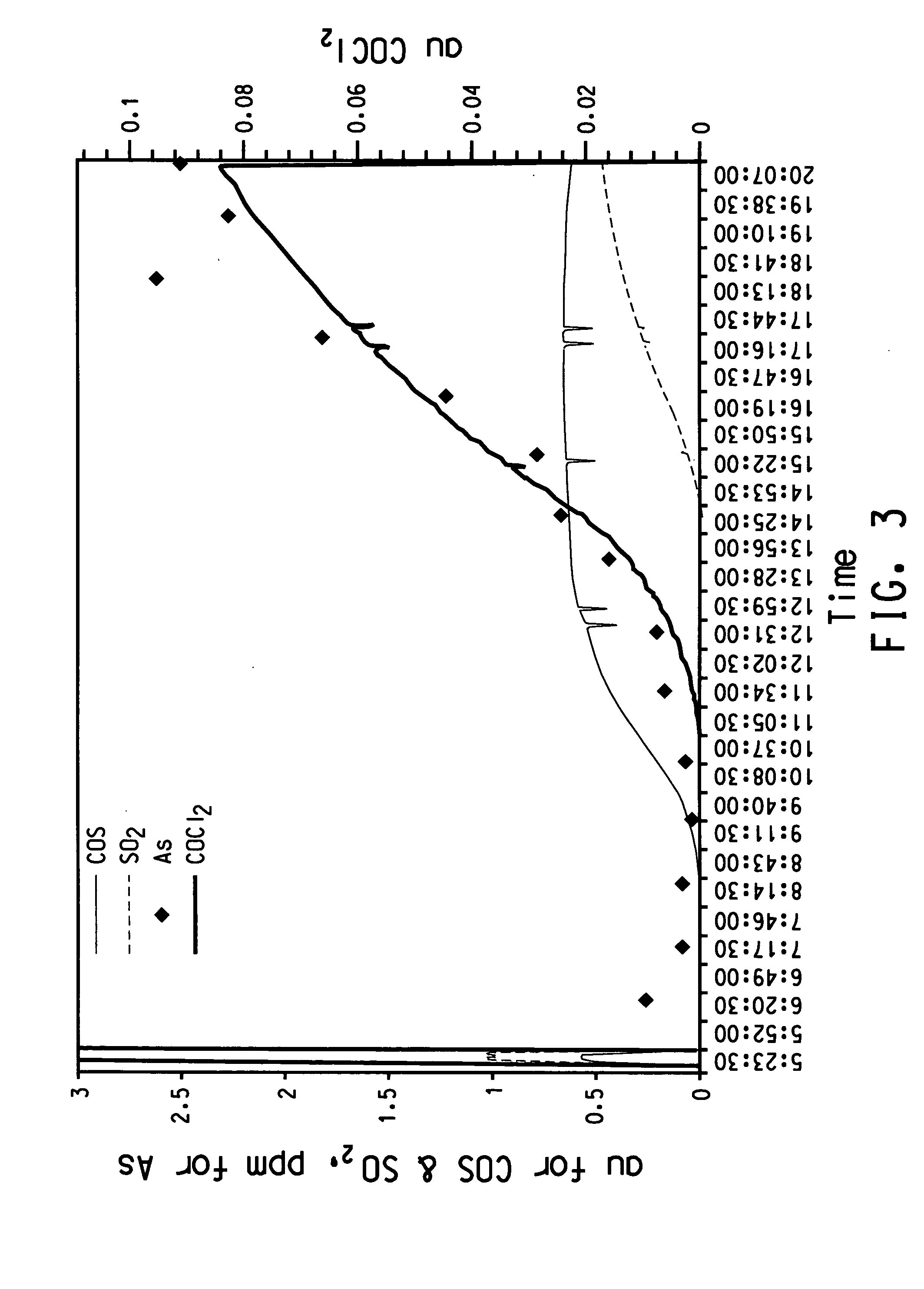
[0050] In this example, the tracker species concentration in the product was measured on a continuous basis using an in-line analyzer. A sample of anhydrous titanium tetrachloride containing 4 ppm arsenic on a titanium tetrachloride basis was used in this Example 2. The feed was also measured by FTIR to contain 0.585 au of COS, 0.147 au of COCl2, and 1.027 au of SO2. The titanium tetrachloride was introduced into the top of the column and allowed to flow by gravity through the column. The titanium tetrachloride collected at the bottom of the column was passed through an FTIR analyzer that analyzed for the presence of the following three tracker species: phosgene, carbonyl sulfide, and sulfur dioxide. FIG. 3 is a plot of absorbance units for each of the three tracker species that shows how the tracker species pattern the concentration of arsenic. FIG. 3 also shows the concentration of tracker species in the feedstock when the feed was passed through the FTIR before the start of the e...
example 3
[0053] In this Example 3, the tracker species concentration was measured in batch samples which were withdrawn from the product and tested off-line.
[0054] A sample of anhydrous titanium tetrachloride containing 33 ppm As on a titanium tetrachloride basis was used in this Example. The feed was also measured by FTIR to contain 1.64 au of CO2 and 0.853 au of CS2. The titanium tetrachloride was introduced into the top of the column and allowed to flow by gravity through the column. The titanium tetrachloride was collected at the bottom of the column. The time averaged product samples were analyzed using an FTIR spectrometer that analyzed for the presence of the following two tracker species: carbon dioxide and carbon disulfide. FIG. 4 is a plot of absorbance units for each of the two tracker species that shows how the tracker species pattern the concentration of arsenic trichloride.
[0055] Table 3 shows the arsenic content in ppm of 6 titanium tetrachloride samples, which are represent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com