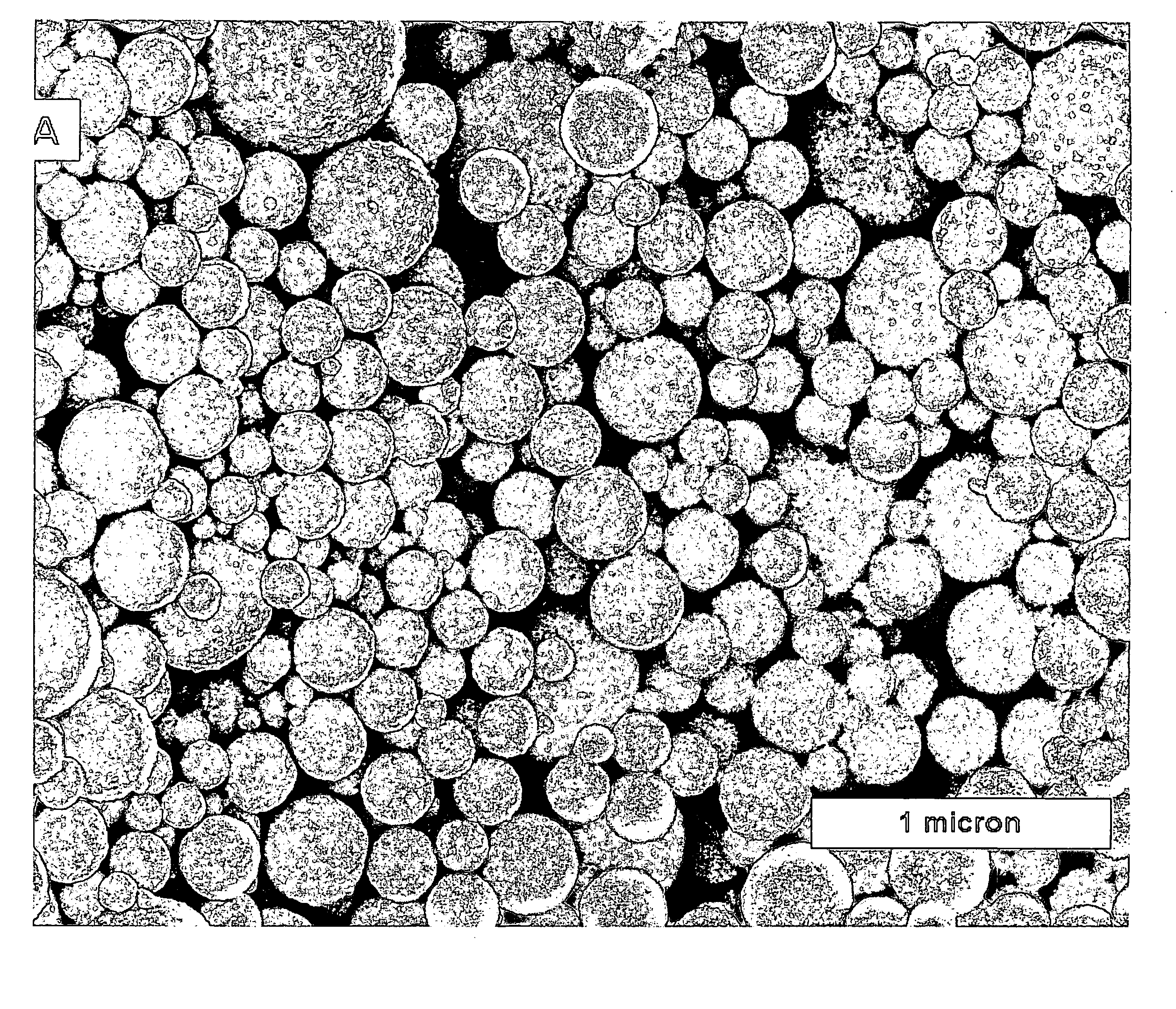
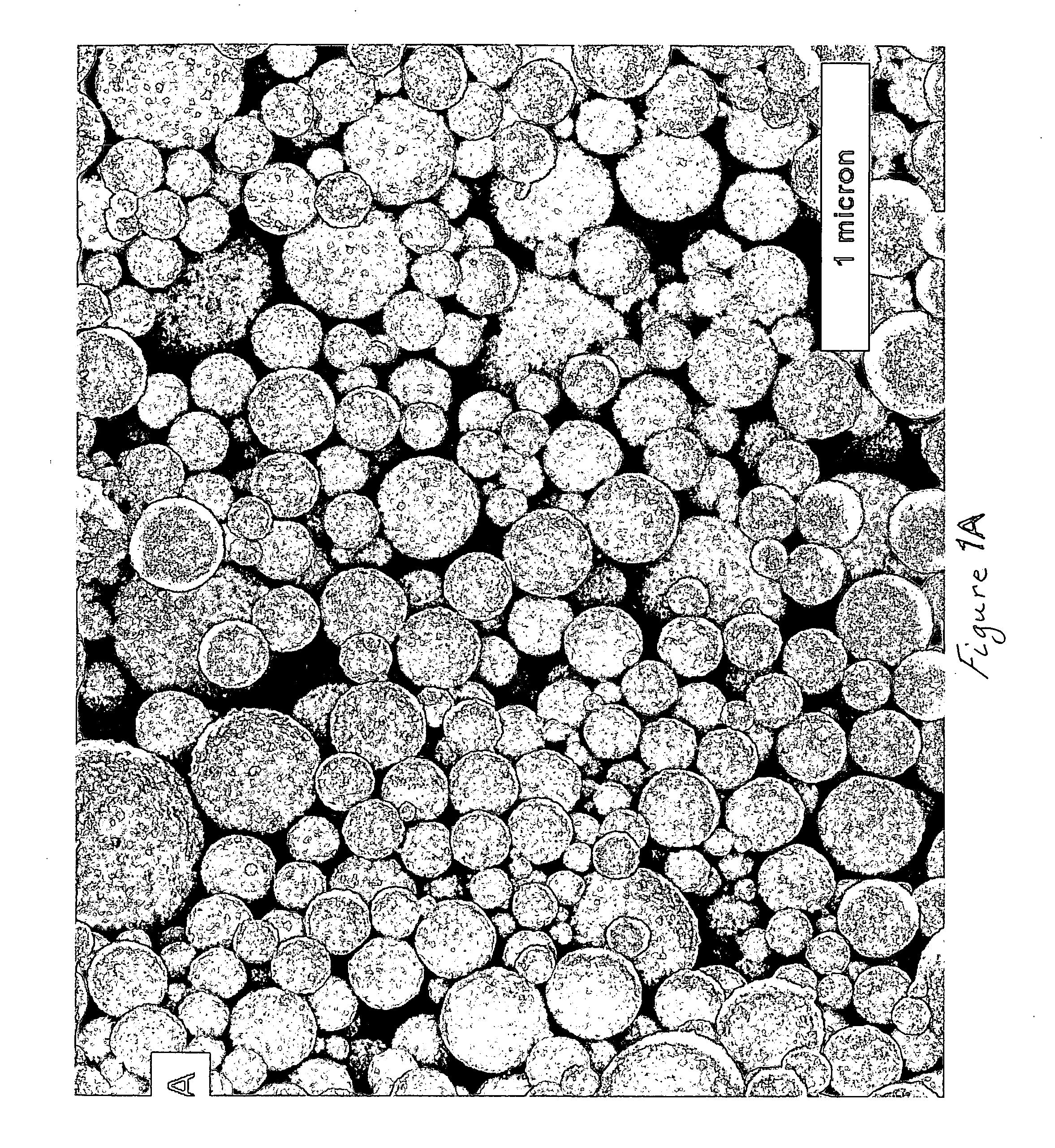
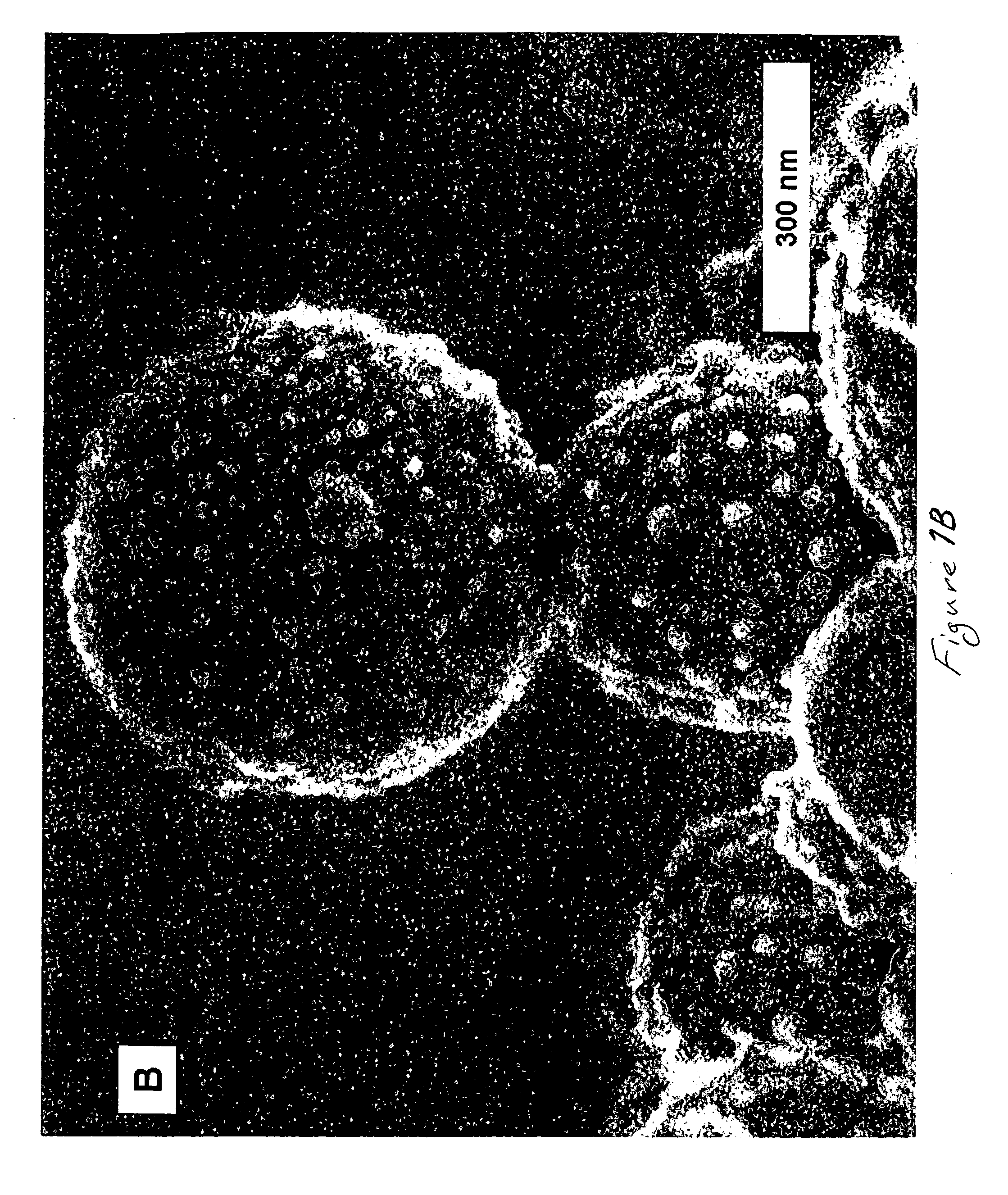
Method of depositing films on aluminum alloys and films made by the method
a technology of aluminum alloy and film, applied in the direction of liquid/solution decomposition chemical coating, surface reaction electrolytic coating, coating, etc., can solve the problems of metal or alloy electroless deposition, metal or alloy capacitance typically degrades, and adverse effects on electrodeposition or electroless deposition, so as to increase the nucleation of discrete metallic nanoparticles thereon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Galvanic Displacement Type Deposition
[0036] In particular, aluminum-copper alloy film covered wafers used in this Example were fabricated as follows: First, a 600-nm layer of SiO2 was thermally grown by steam oxidation of each silicon wafer. Second, a 3 -μ m thick layer Al-Cu alloy (99.5 weight % aluminum and 0.5 weight % copper) was deposited on the layer of Sio2 by physical vapor deposition (PVD). Third, each wafer having the Al-Cu alloy layer was anodized in an electrochemical cell at 50 V dc for 20 min in 3% by weight oxalic acid aqueous solution at 0° C. The electrical contact was made to the top metallic layer outside the electrochemical cell. The steady state current density, established after 5 minutes of anodization, was approximately 1.4 mA / cm . Preliminary experiments revealed that the entire 3 μ m thick metallic layer was anodized in approximately 80-85 min. Thus, anodization for 20 min consumed about 0.75 μ m of the metallic layer. Following anodization, the porous and...
example 2
[0055] In particular, aluminum-copper alloy covered wafers used in this Example were fabricated as follows: First, silicon wafers with a 600 nm thick layer of SiO2 overlayed with a 3 μ m thick layer of 99.5 wt % Al and 0.5 wt % Cu (deposited by physical vapor deposition) were used in all experiments. The Al-Cu alloy layer was anodized with a Pt mesh counter electrode at 50 V DC for 20 min in 3 wt % H2C2O4 acid and at 0° C. The electrical contact was made to the top metallic layer outside the electrochemical cell. After anodization, the porous and barrier layers of aluminum oxide were etched in a mixture of 0.4 M H3PO4 and 0.2 M H2CrO4 acids at 50 ° C. for approximately 90 minutes to remove the outer porous aluminum oxide and partially remove the thickness of the barrier aluminum oxide. Upon completion of etching the specific capacitance of barrier aluminum oxide was determined to be 5.8 μ F / cm2. Assuming that the dielectric constant was 8.6, the layer of barrier aluminum oxide remai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle diameter | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com