Plasmodium falciparum AMA-1 protein and uses thereof
a technology of plasmodium falciparum and ama-1, which is applied in the field of purified plasmodium falciparum protein, can solve problems such as problematic prokaryotic expression of ama1 from various species, and achieve the effect of optimal reactivity and elimination of contaminating proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
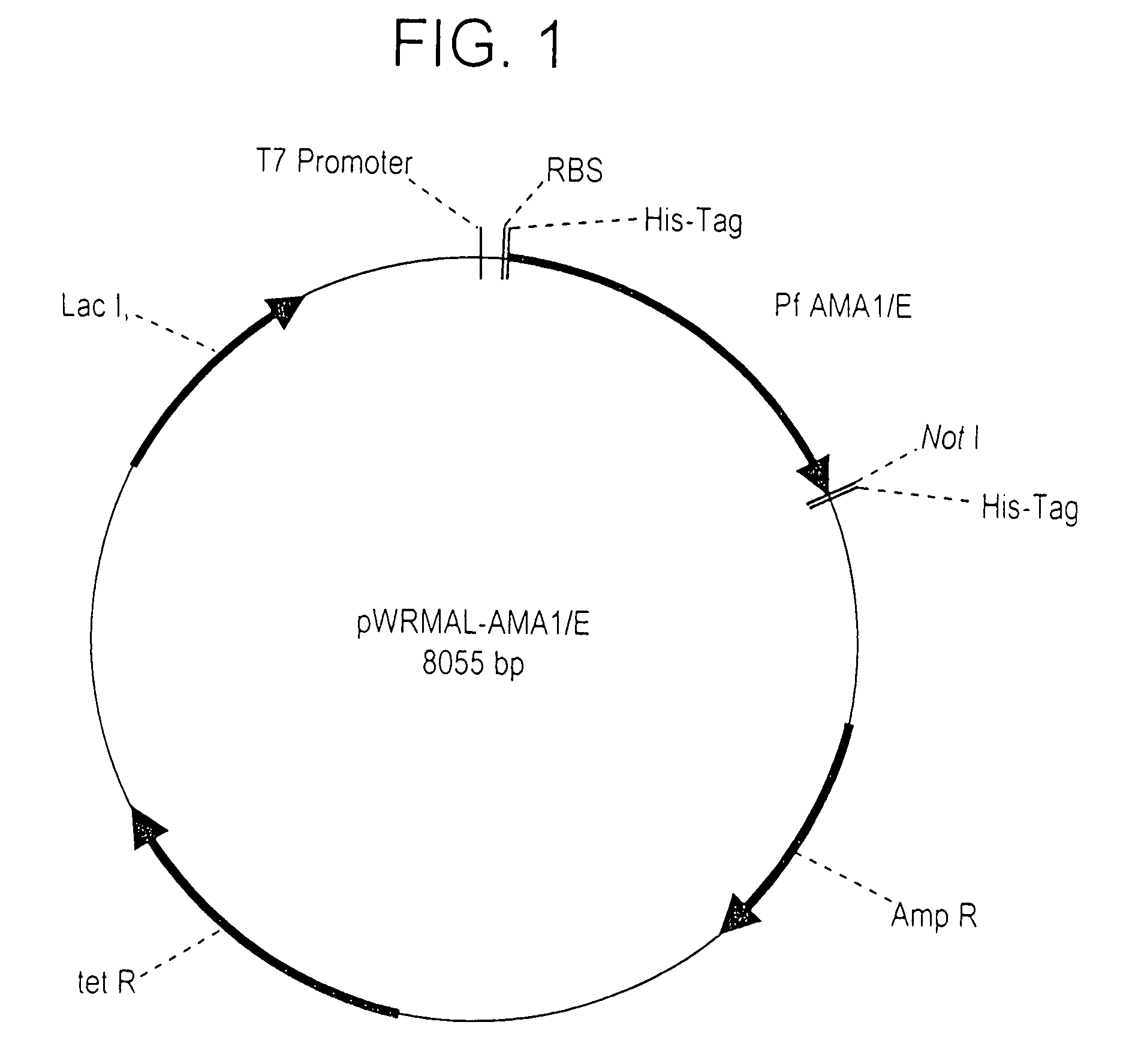
[0194] Fermentation of E. coli Origami (DE3) expressing the r-AMA1 / E protein at 10 L and 300 L scale: The synthetic gene cloned in the vector pWRMAL was sequenced and the translation of this gene sequence revealed no amino acid changes from the published 3D7 clone sequence (GenBank™ Accession No U65407.1). Fermentation conditions were developed in a 10 L bioreactor and later scaled-up to a 300 L GMP fermentation. The 10 mL fermentation routinely resulted in 150 gm cell paste while the 300 L fermentation resulted in 4.5 kg cell paste. The final plasmid stability for the GMP fermentation was 36%. Although the use of Origami (DE3) increased the proportion of r-AMA1 / E in the soluble fraction (compared to the conventional BL21 (DE3) strain), protein fractionation experiments showed that a majority of r-AMA1 / E was still localized in the insoluble fraction (data not shown).
example 2
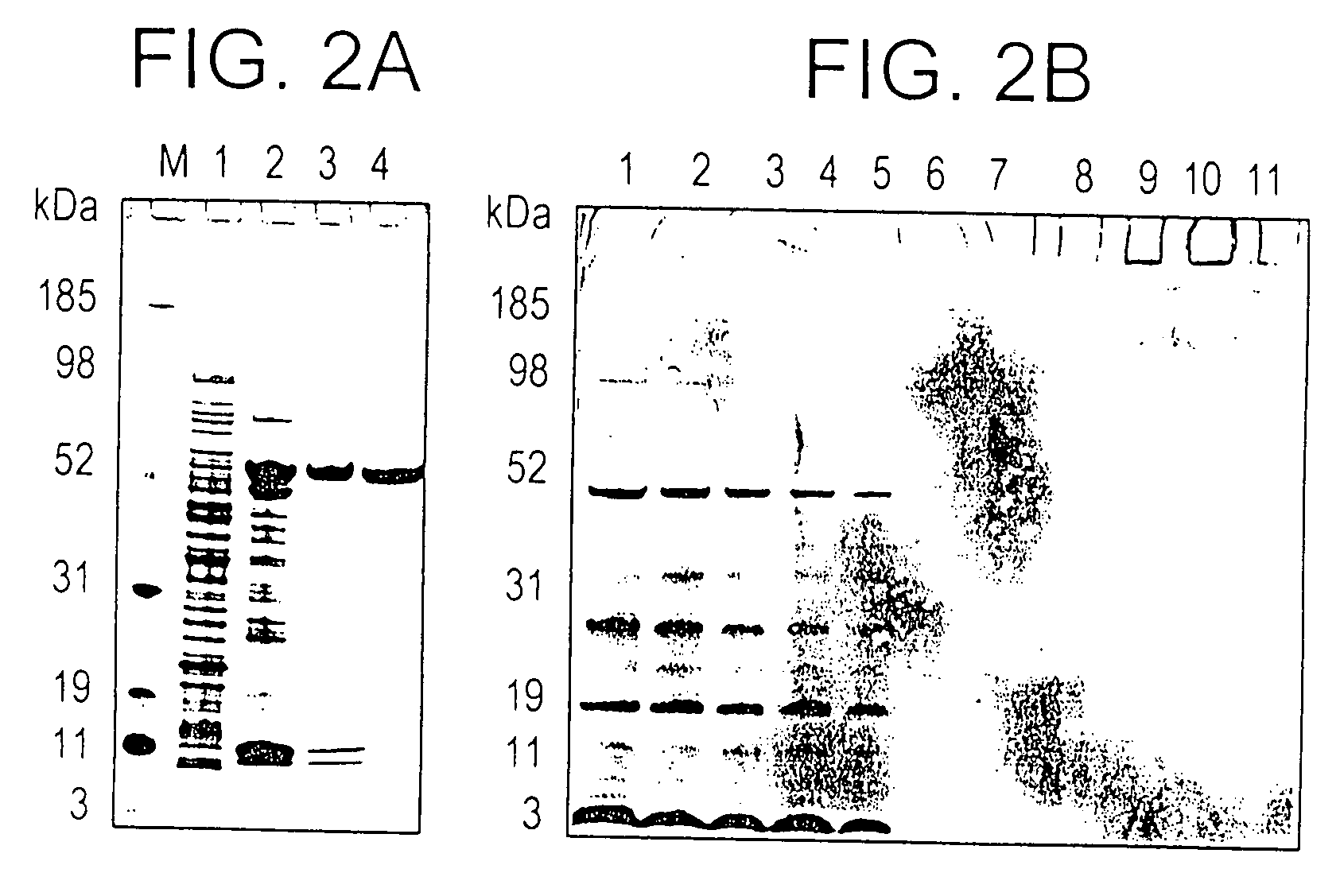
[0195] Extraction of r-AMA1 / E in sarkosyl and its enrichment by Ni+2 affinity chromatography: Aliquots were taken from the GMP cell paste lot and a scalable refolding and purification process was developed. During cell lysis soluble and insoluble forms of r-AMA1 / E were extracted with buffer containing 5% sarkosyl. The r-AMA1 / E constituted ˜1-2% of total cell protein estimated by laser densitometry of a SDS-PAGE run under reduced conditions (FIG. 2A, lane 1). Following the first step of purification over Ni+2 column, r-AMA1 / E was enriched to ˜40% of total protein (FIG. 2A, lane 2). A large fraction of r-AMA / E present in the Ni+2 elution, was aggregated as seen on a non-reduced SDS-PAGE (data not shown).
example 3
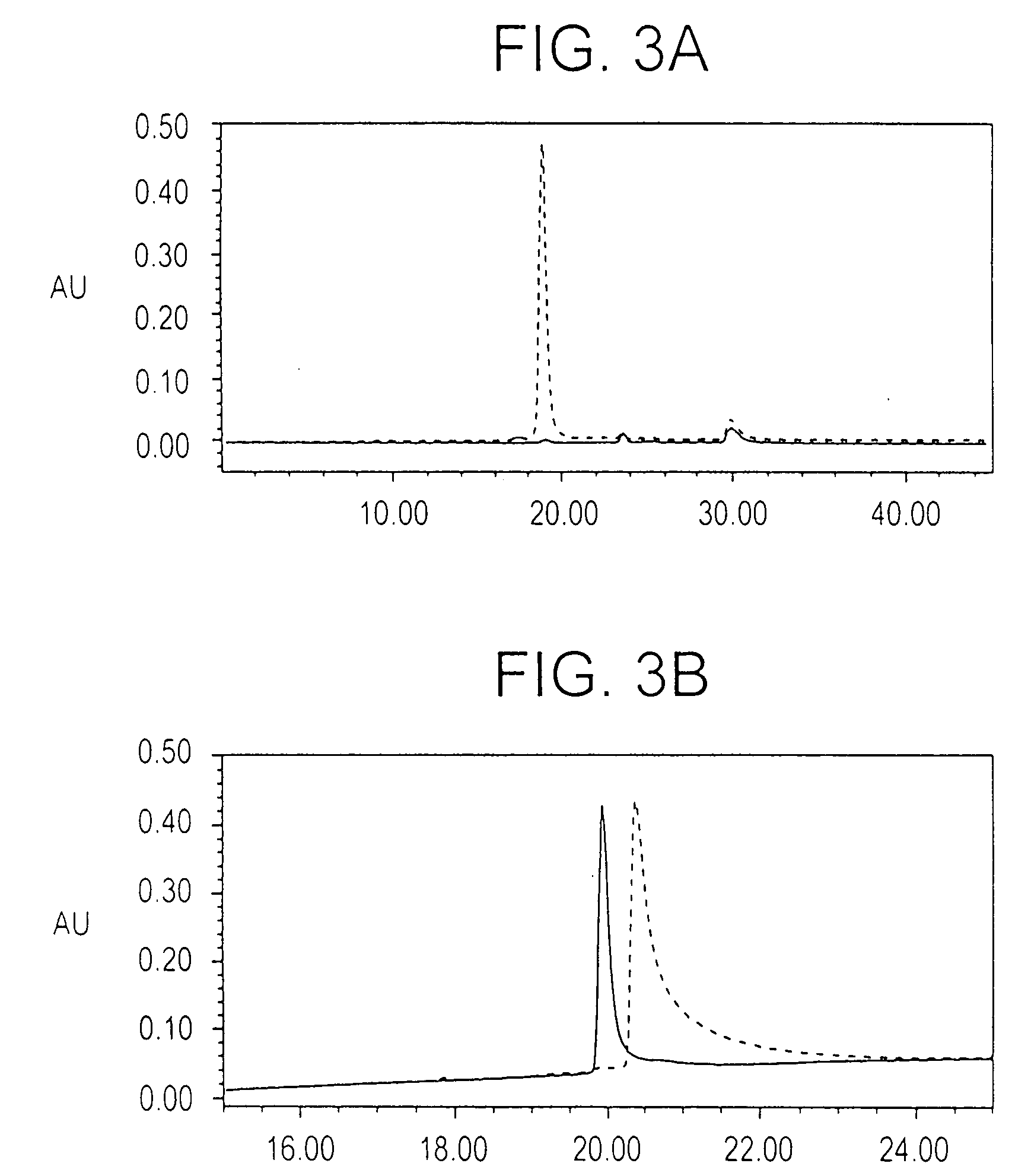
[0196] Optimization of the refolding conditions: In order to find the optimal refolding conditions, the Ni+2 elution was subjected to rapid dilution in refolding buffers of varying GSH / GSSG ratios, at pH 8.0, in phosphate buffer. Serial dilutions of these test refolding mixtures were coated on a microtiter plate and ELISA reactivity against the conformation specific, inhibitory, monoclonal antibody 4G2dc1, was used as a measure of folding efficiency; while the reactivity to a monoclonal anti-hexa-histidine antibody was used to confirm equivalent coating efficiency. Ratios of GSH / GSSG tested for refolding included 1 / 0.1 mM, 1 / 0.25 mM, 1 / 1 mM, and 0.1 / 1 mM respectively, while phosphate buffer containing EDTA (pH 8.0) alone was used as a control. The GSH / GSSG ratios of 1 / 0.1 mM and 1 / 0.25 mM were found to be equally efficient, both of which gave 4G2dc1 reactivity about 5 times higher than the phosphate buffer control. As the GSH / GSSG ratio of 1 / 0.25 mM had been previously reported for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| apparent molecular weight | aaaaa | aaaaa |
| apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com