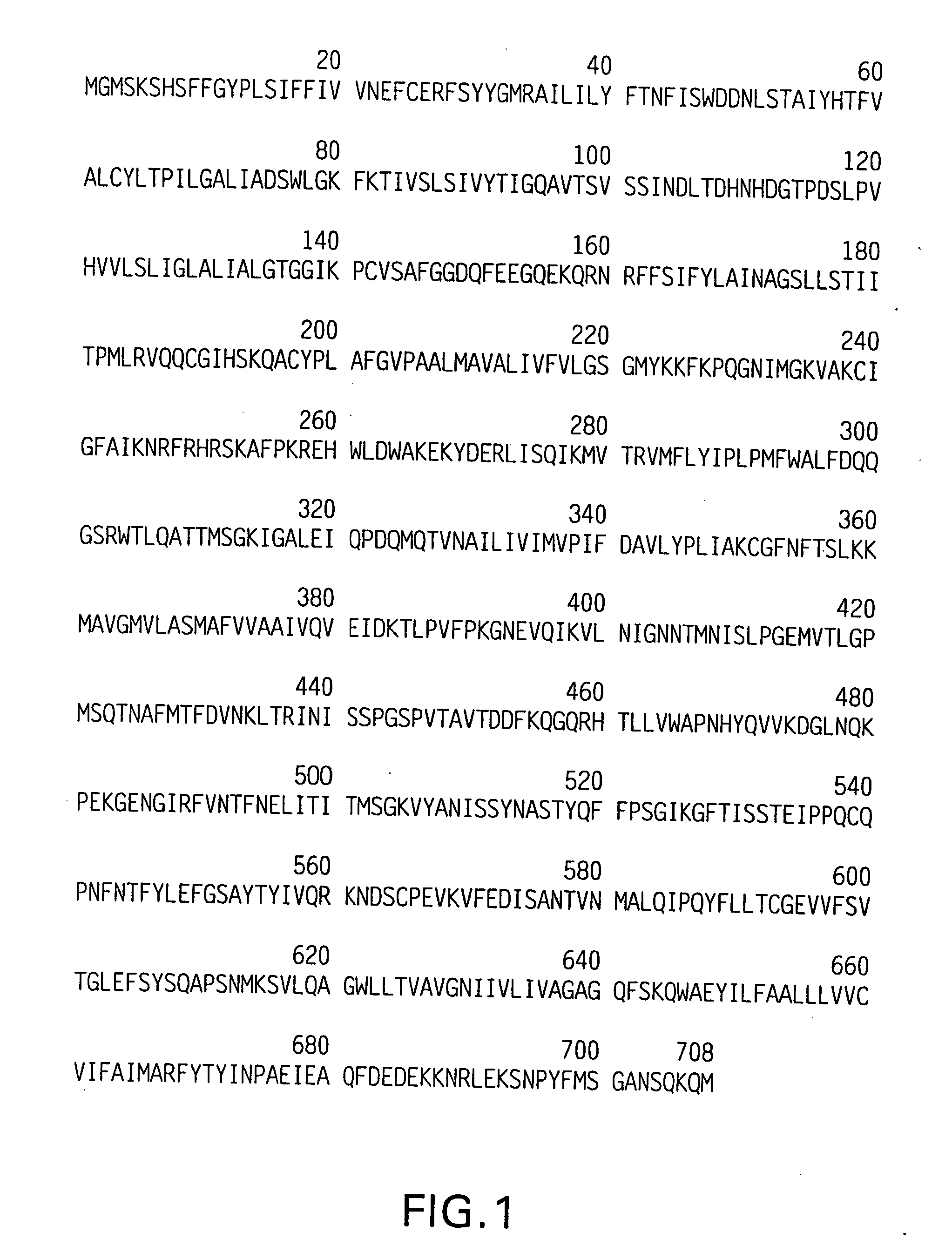
Random peptides that bind to gastro-intestinal tract (GIT) transport receptors and related methods
a technology of git and transport receptor, which is applied in the direction of peptide/protein ingredients, peptide sources, metabolic disorders, etc., can solve the problems of inability to carry out intravenous drug administration, risk of adverse effects, and risk of infection at the site of repeated injections, so as to facilitate its absorption into the systemic system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Peptide Added at the Final Washing Stage
[0338] Product: Bovine Insulin loaded nanoparticles
[0339] Aim: To prepare a 2 g batch of insulin loaded nanoparticles at a theoretical loading of 50 mg / g and with the peptide ZElan018 added.
Formulation DetailsRG504H (Lot no. 250583)2.0gAcetone45mlEthanol:5mlPVA (aq. 5% w / v)400mlBovine Insulin (Lot no. 86H0674)100mgPeptide: PAX2 (ZElan018)10 mg / 50 mldH2O
Experimental details:
[0340] The 5% w / v PVA solution was prepared by heating water until near boiling point, adding PVA and stirring until cool. The organic phase was prepared by adding acetone, 45 ml, and ethanol, 5 ml, together. The polymer solution was prepared by adding RG504H, 2 g, to the organic phase and stirring until dissolved. The IKA™ reactor vessel was set up, all seals greased and the temperature was set at 25° C. The PVA solution, 400 ml, was added into the reactor vessel and stirred at 400 rpm.
[0341] Bovine insulin, 100 mg, was added into the stirring PVA solution. Using cle...
example 2
Peptide Added at the Beginning of Manufacture
[0345] Product: Bovine Insulin loaded nanoparticles
[0346] Aim: To prepare a 2 g batch of insulin loaded nanoparticles at a theoretical loading of 50 mg / g and with the peptide ZElan018 added at the beginning of manufacture.
Formulation DetailsRG504H (Lot no. 250583)2.0gAcetone45mlEthanol:5mlPVA(aq. 5% w / v)400mlBovine Insulin (Lot no. 65H0640)100mgPeptide: PAX2 (ZElan018ii)10mg
Experimental Details:
[0347] The 5% w / v PVA solution was prepared by heating water until near boiling point, adding PVA and stirring until cool. The organic phase was prepared by adding acetone, 45 ml, and ethanol, 5 ml, together. The polymer solution was prepared by adding RG504H (polyactide-co-glycolide, Boehringer Ingelheim), 2 g, to the organic phase prepared in step above and stirring until dissolved. The IKA™ reactor vessel was set up, all seals greased and the temperature was set at 25° C. The PVA solution, 400 ml, was added into the reactor vessel and stir...
example 3
Peptide Added 1 Hour before Centrifugation
[0351] Product: Bovine Insulin loaded nanoparticles
[0352] Aim: To prepare a 1 g batch of insulin loaded nanoparticles at a theoretical loading of 50 mg / g and with the peptide ZElan018 added 1 hour before centrifugation.
Formulation DetailsRG504H (Lot no. 250583)1.0gAcetone22.5mlEthanol:2.5mlPVA(aq. 5% w / v)200mlBovine Insulin (Lot no. 65H0640)50mgPeptide: PAX2 (ZElan018)5mg
Experimental Details:
[0353] The 5% w / v PVA solution was prepared by heating water until near boiling point, adding PVA and stirring until cool. The organic phase was prepared by adding acetone, 22.5 ml, and ethanol, 2.5 ml, together. The polymer solution was prepared by adding RG504H, 1 g, to the organic phase prepared above and stirring until dissolved. The IKA™ reactor vessel was set up, all seals greased and the temperature was set at 25° C. The PVA solution, 200 ml, was added into the reactor vessel and stirred at 400 rpm.
[0354] Bovine insulin, 50 mg, was added in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com