Circular dumbbell decoy oligodeoxynucleotides (cdodn) containing dna bindings sites of transcription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibitory Effects of Novel AP-1 Decoy Oligodeoxynucleotides on Proliferation of Vascular Smooth Muscle Cells In Vitro and Neointima Formation In Vivo
Materials and Methods
Animals
[0130] Nine- to ten-week old male Sprague-Dawley rats (Hyochang, Taegu, Korea) weighing 280 to 320 g were used. All procedures were in accordance with institutional guidelines for animal research.
Cell Culture
[0131] Human VSMC were harvested as described in Ahn et al 2001 supra, and rat aortic smooth muscle cells w re harvested from the thoracic aorta of adult male Sprague-Dawley rats (200-250 g). VSMC were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, N.Y., USA) containing 20% fetal bovine serum (Gibco BRL). VSMC purity was characterized by positive staining with smooth muscle specific α-actin monoclonal antibodies (Sigma, St. Louis, Mo., USA).
Construction of CDODN
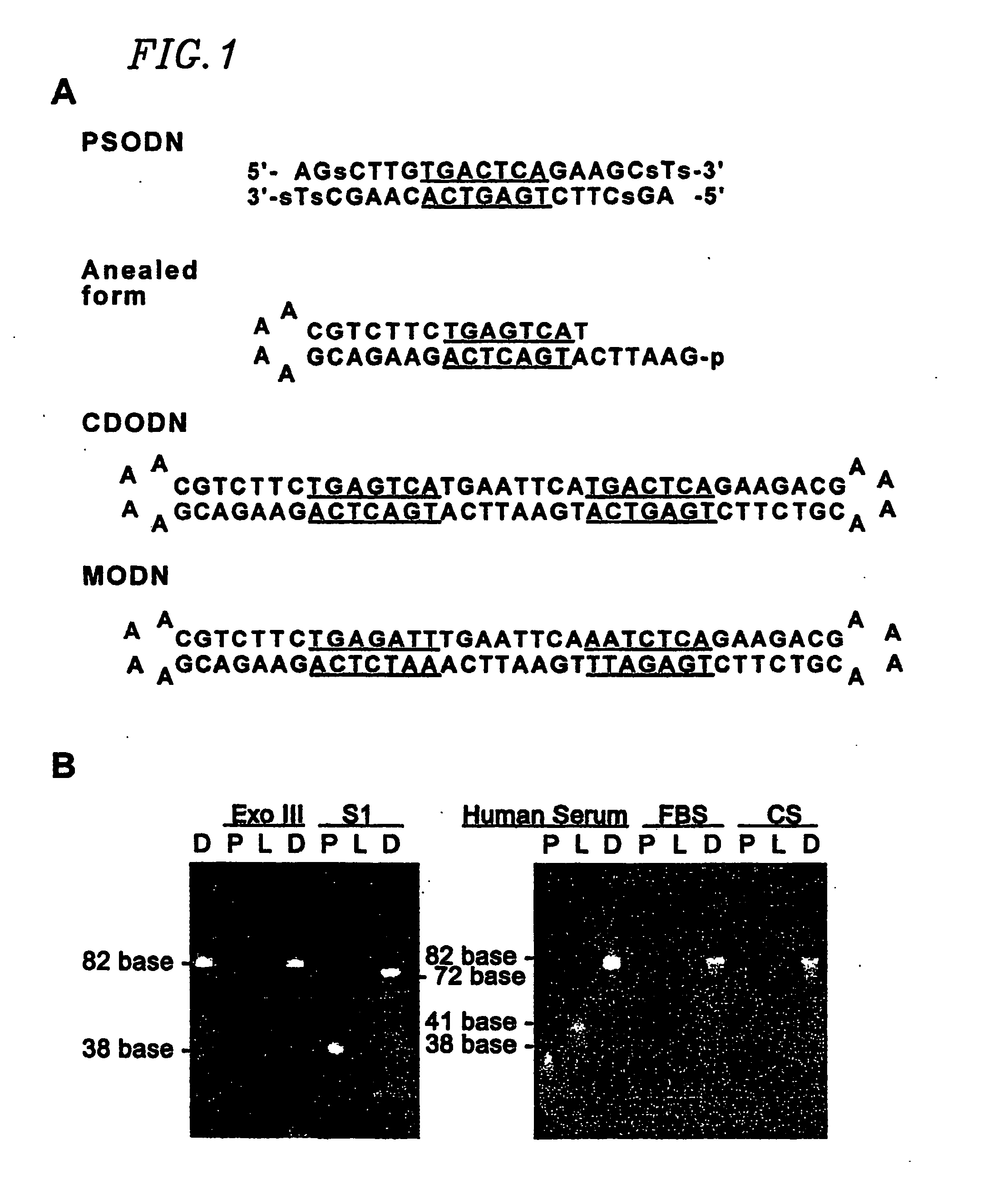
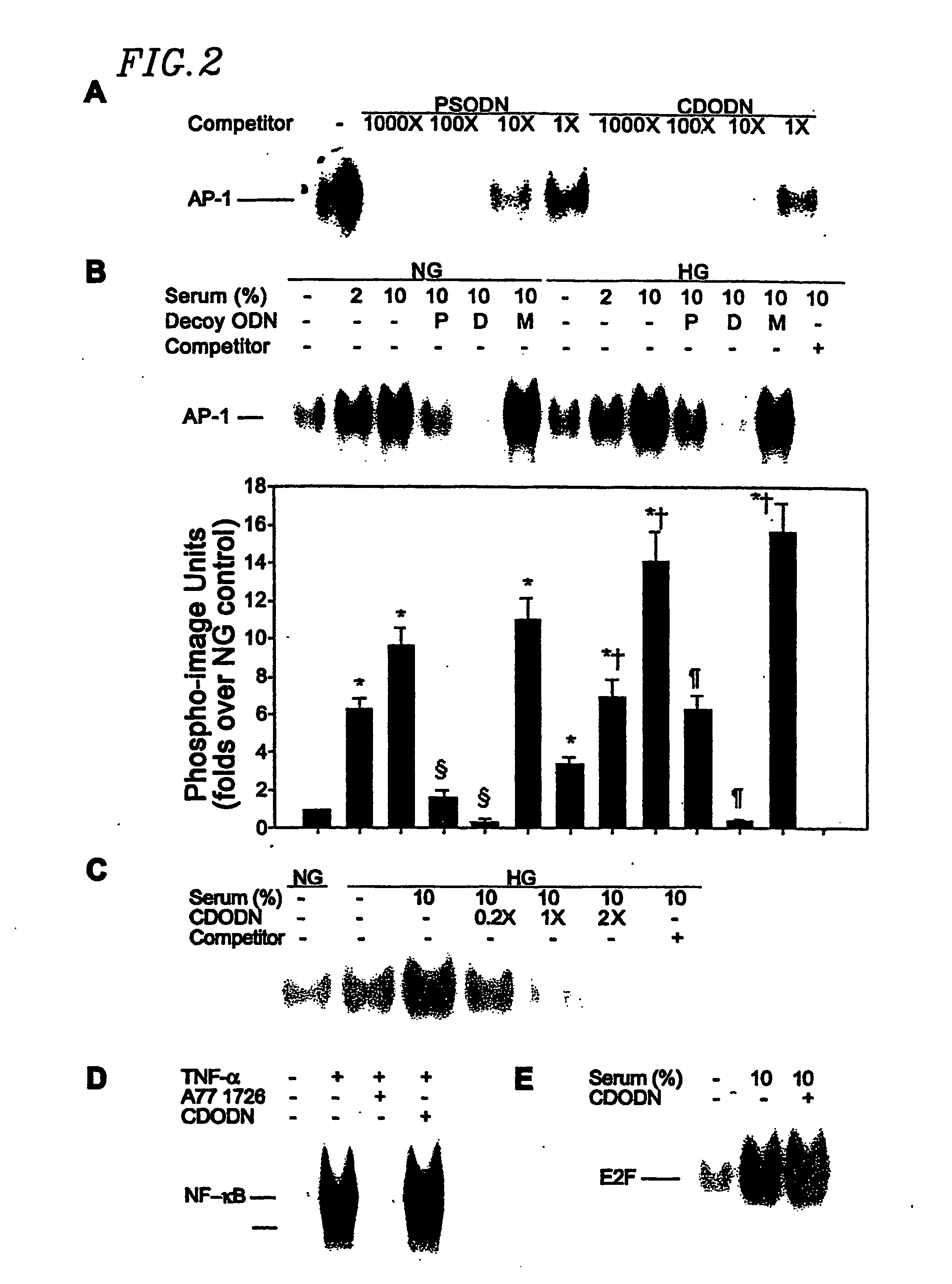
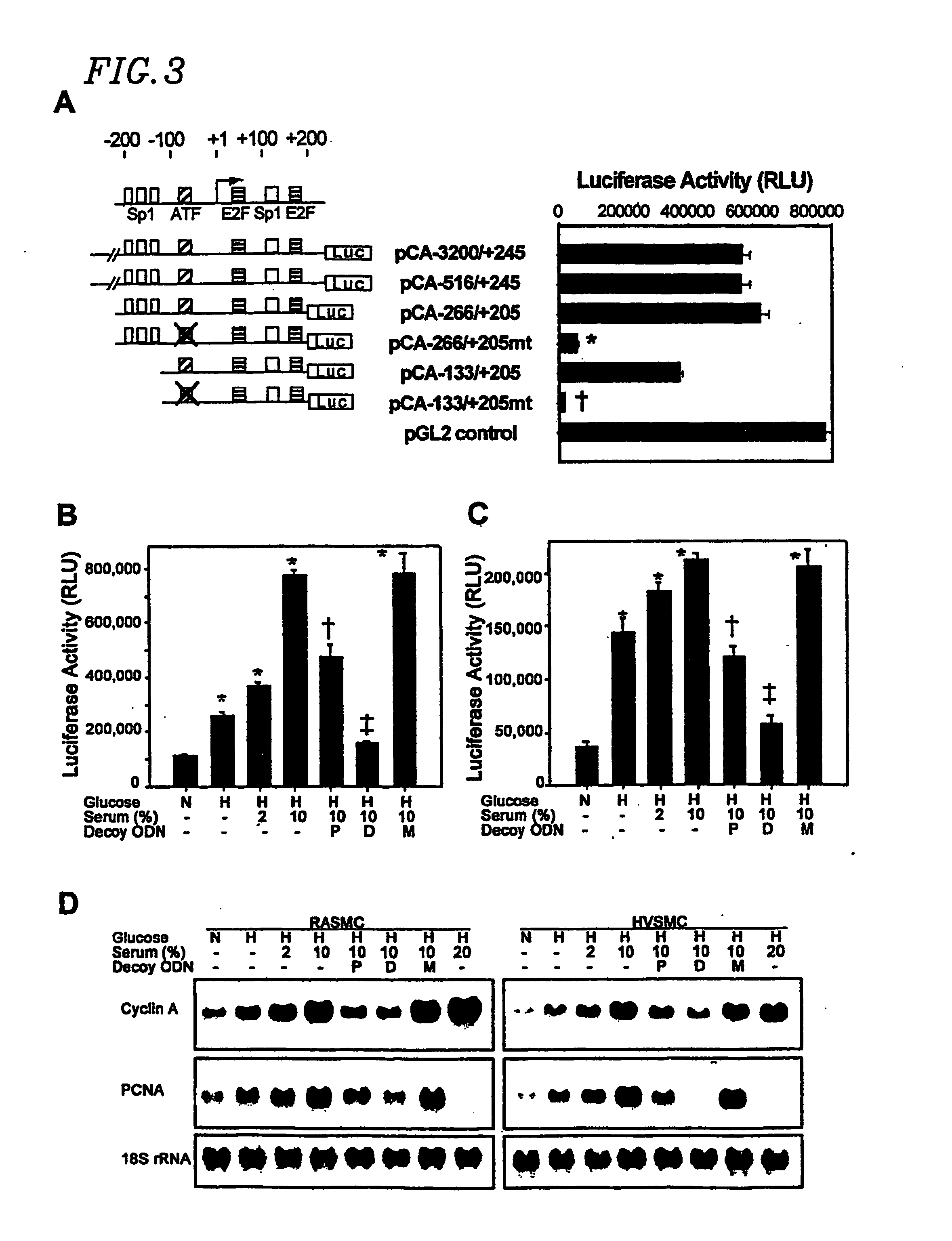
[0132] The sequences of dumbbell type and phosphorothioate double-stranded ODN derived from the AP-1 b...
example 2
Effects of Novel E2F Decoy Oligodeoxynucleotides
Materials and Methods
Animals
[0155] Nine- to ten-week old male Sprague-Dawley (SD) rats weighing 280 to 320 g were used. All procedures were in accordance with institutional guidelines for animal research.
[0156] Human VSMCs were isolated from thoracic aortas of heart transplant donors. The collection of this tissue was approved by the Ethics Committee of the institution. Rat VSMCs were harvested from thoracic aortas of adult male SD rats. VSMCs were cultured in DMEM (Gibco BRL, Grand Island, N.Y., USA) containing 20% FBS (Gibco BRL). VSMC purity was characterized by positive staining with smooth muscle specific α-actin monoclonal antibodies (Sigma, St. Louis, Mich., USA).
[0157] After reaching 80-90% confluence in 100-mm dishes, human VSMC were serum-starved for 24 h in serum free medium, and were subjected to either control normal glucose medium (DMEM containing 5.5 mmol / l D-glucose) or conditioned medium (DMEM cont...
example 3
Effects of Novel NF-κB Decoy Oligodeoxynucleotides
Construction of Dumbbell Type Decoy ODN
[0178] The sequences of dumbbell type and phosphorothioated double-stranded ODN against NFκB binding site and mutated ODN used in this invention are as follows: CD-NF (note; consensus sequences are underlined), 5′-GGATCCGGGGATTTCTATTGCAAAAGCAATAGCGCGAAAC-3′ (SEQ ID NO: 15); phosphorothioate NFκB decoy (PS-NF), 5′-ATsTTAAGGGGATTTCCCTTTCTCAsAs-3′ (SEQ ID NO: 16); mutated E2F decoy (M-NF), 5′-GGATCCGGGGATATTTATTGCAAAAGCAATAAATCGAAAC-3′ (SEQ ID NO: 17). CD-NF was anticipated to form a stem-loop structure. The stem is formed by complementary sequences at both ends of each oligo. The 5′ terminus of the stem has 6 bases of a single-stranded sequence of 5′-GGATCC-3′ as enzyme site of BamHI. Two oligo molecules were joined by the complementary 6 base sequences at both 5′ ends. ODN were annealed for 2 h, while the temperature descended from 80° C. to 25° C. One unit of T4 DNA ligase was added and incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com