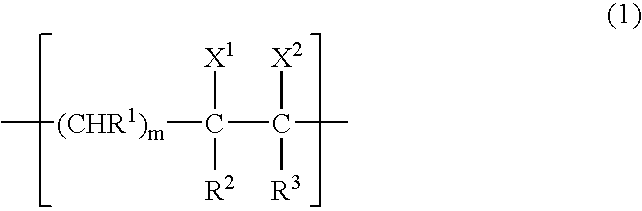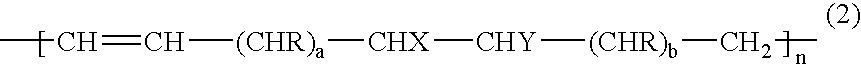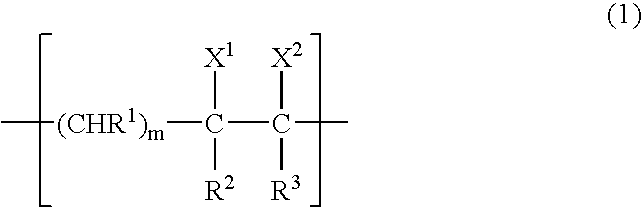Gas-barrier resin composition
a technology of resin composition and gas barrier, which is applied in the field of resin composition and film, can solve the problems of inviting higher cost, and achieve the effects of high gas barrier properties, low crystallization rate, and high cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
referential example 1
(a) Preparation of Poly(5-cyclooctene-1,2-diol)
[0204] In a 3-L separable flask equipped with a thermometer, dropping funnel, reflux tube and stirrer were placed 5-cyclooctene-1,2-diol (320 g, 2.25 mol), 3-cis-hexen-1-ol (2.0 g, 0.02 mol) as a chain transfer agent and tetrahydrofuran (1280 g), and the resulting solution was held to 55° C. To the stirred solution was added dropwise a solution of 1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene(tricyclohexylphosphine)benzylideneruthenium dichloride (0.127 g, 0.15 mmol) as a ring-opening metathesis polymerization catalyst in tetrahydrofuran (5 mL). Thirty minutes later, a solution of ethyl vinyl ether (2 g, 0.028 mol) as a terminating agent in a mixture of methanol (500 g) and tetrahydrofuran (250 g) was added, followed by stirring at room temperature. The reaction mixture was poured into hexane (20 L), the precipitate was separated and recovered by filtration, and the solvent was distilled off under reduced pressure to yie...
example 1
[0206] The hydrogenated derivative of poly(5-cyclooctene-1,2-diol) (99 parts by weight) prepared in Referential Example 1(b) and an ethylene-vinyl alcohol copolymer (ethylene unit content: 38 percent by mole, saponification degree: 99.9%, viscosity-average polymerization degree: 1,150) (1 part by weight) were melted and kneaded at 170° C. at 100 rpm for 10 minutes using a Laboplast mill (product of Toyo Seiki Seisaku-sho, Ltd.) and thereby yielded a resin composition. The crystallization temperature (Tcc), half time of crystallization (t1 / 2) and oxygen permeability of the resin composition were determined by the above procedures. The results are shown in Table 1.
example 2
[0207] A resin composition was prepared by the procedure of Example 1, except for using an ethylene-vinyl alcohol copolymer (ethylene unit content: 38 percent by mole, saponification degree: 99.9%, viscosity-average polymerization degree: 750) (1 part by weight) instead of the ethylene-vinyl alcohol copolymer (ethylene unit content: 38 percent by mole, saponification degree: 99.9%, viscosity-average polymerization degree: 1,150) (1 part by weight). The crystallization temperature (Tcc), half time of crystallization (t1 / 2) and oxygen permeability of the resin composition were determined by the above procedures. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mol % | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| half time of crystallization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com