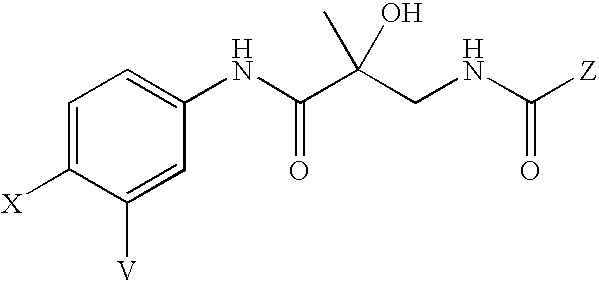
Novel skin care compositions
a composition and skin technology, applied in the field of cosmetic compositions, can solve the problems of few systematic studies to support such claims, adverse effects on skin metabolism, and many side effects at the level of efficacy, and achieve the effect of reducing the toxicity of products, preserving bacteria efficiently, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Typical Composition 100 g of the Novel Skin Care Cosmetic Preparation
[0066] 0.5 g extract from Japanese knotweed (min. 95% trans-resveratrol); 3,4′,5-trihydroxy-trans-stilbene, CAS No.: [501-36-0]
[0067] 0.5 g extract from frankincense (Olibanum)—Boswellia serrata (60-70% triterpenes) CAS No.: [8050-07-5]
[0068] 0.5 g extract from vegetable oil Brassica oleifera / Glycine soja (unsaponifiable fractions CAS [91770-67-1]
[0069] 3.0 g vitamin E (DL-α-tocopherol acetate); 3,4-dihydro-2, 5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-ol acetate, 3.000 IU CAS No.: [7695-91-2]
[0070] 1.2 g vitamin A (retinol palmitate); 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl) 2,4,6,8-nonatetraen-1-ol palmitate, 2,000.000 IU, CAS No.: [79-81-2]
[0071] 2.0 g fluridil CAS No.: [260980-89-0]
[0072] 0.1 g BHT (butylhydroxytoluene), 2,6-Di-tert-butyl-4-methylphenol CAS No.: [128-37-0]
[0073] 5.0 g rubbing alcohol (isopropanol), CAS No.: [67-63-0]
[0074] 40.0 g refined oil from grape seed, ...
example 2
Ocular Toxicity: Effects on the Ocular Fibroblasts
[0076] Rabbit corneal fibroblasts, to which Neutral Red is added in vitro, are exposed to the product at 15, 25 and 50% concentrations for I minute. The cell viability is measured by Optical Density. The culture medium serves as negative control. IC50 less than 50% was found, indicating that the product has a very low toxicity towards ocular mucous membranes, and is equivalent to the marketed products of the same category.
example 3
Evaluation of the Antimicrobial Properties
[0077] The test, according to USP XXV, is based on inoculating the product with 5 strains, i.e., S. aureus, P. aeruginosa, E. coli, C. albicans and A. niger, incubation at 20° C. with the counts performed at 7, 14, 21 and 28 days. The antimicrobial (“preserving”) efficiency of the product is considered positive when at day 14 the bacterial counts (measured by concentration CFU / g) are reduced by at least 2 logs and in the following 14 days continue to drop or stay equal to the starting counts. For yeasts / moulds the counts must be equal or lower at day 14 and 28, compared to the initial count.
TABLE 3CONCENTRATIONS MEASURED (CFU / g)SOUCHES / STRAINSINOCULUMJ7 / D7J14 / D14J21 / D21J28 / D28S. aureus1.90E+070.00E+000.00E+000.00E+000.00E+00P. aeruginosa2.30E+070.00E+000.00E+000.00E+000.00E+00E. coli1.10E+070.00E+000.00E+000.00E+000.00E+00C. albicans2.20E+070.00E+000.00E+000.00E+000.00E+00A. niger3.10E+070.00E+000.00E+000.00E+000.00E+00
[0078] The product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com