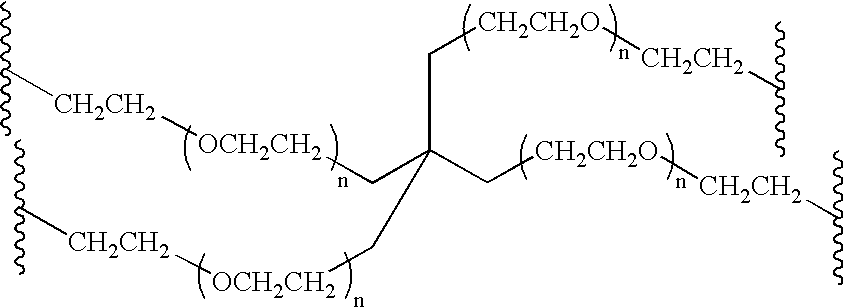
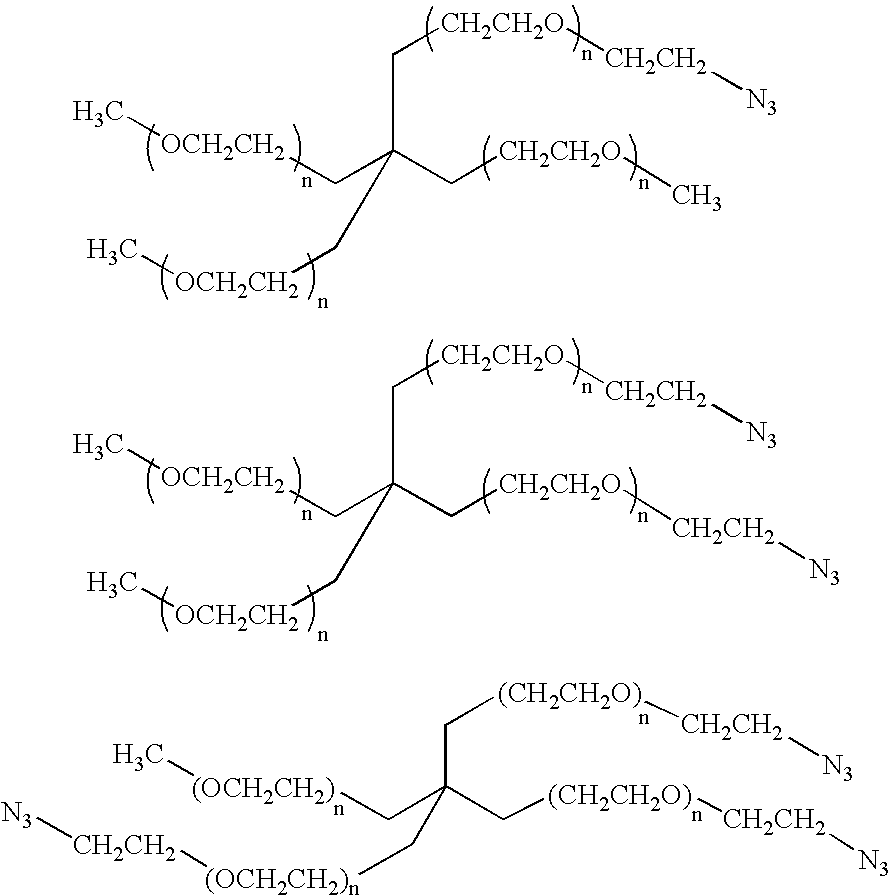
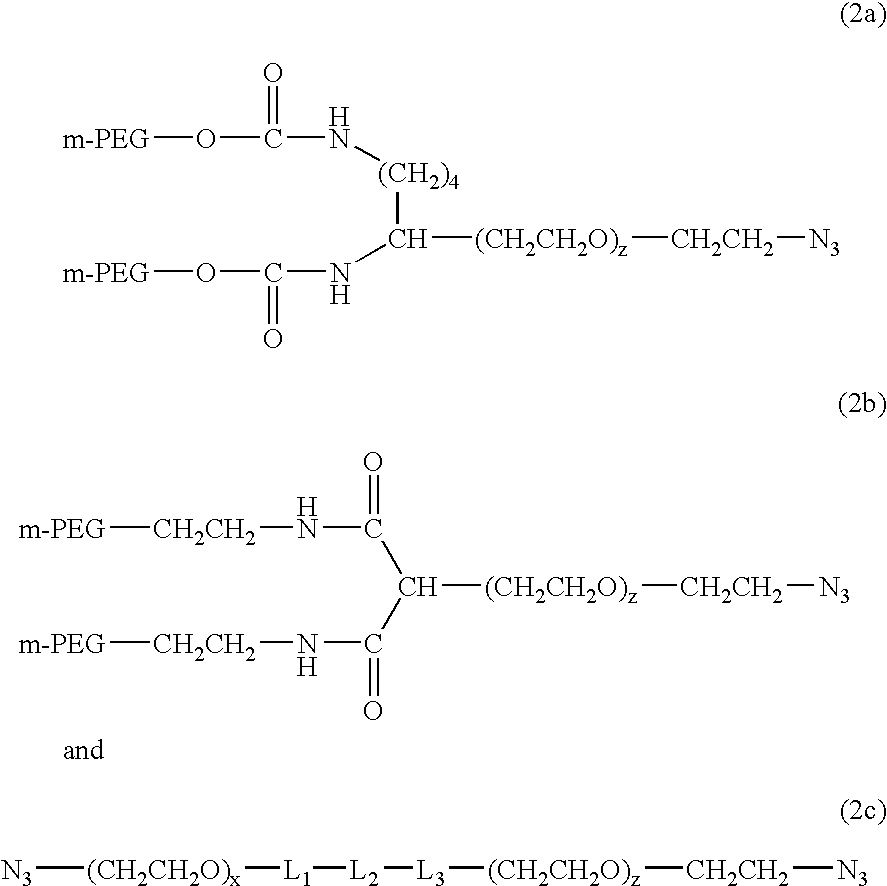Methods of preparing polymers having terminal amine groups
a technology of terminal amine group and polymer, which is applied in the field of preparing activated polymers, can solve the problems of significant percentage of peg-halide hydrolysis, significant reduction of purity of the desired end product, and significant increase in the cost of the desired product, so as to achieve high purity and reduce cost. , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0063]
PEG-tosylate (PEG-OTs).
Method A. A solution of 20KDaΔPEG-OH (i.e. bis-PEG-OH) (2.8 g, 0.14 mmol) was azeotroped for 2 hours in toluene. The toluene was removed under vacuum and the solid residue was redissolved in 50 mL of anhydrous dichloromethane (DCM). To this solution was added triethylamine (195 μL, 1.4 mmol) and 4-dimethylaminopyridine (DMAP) (165 mg, 1.35 mmol). The mixture was cooled in an ice bath and p-toluenesulfonyl chloride (267 mg, 1.4 mmol) in DCM was then added dropwise. The reaction mixture was gradually warmed to room temperature and stirred overnight. The reaction mixture was washed twice with a 0.1 N HCl solution. The organic phase was evaporated under vacuum. The resulting solid was dissolved in the minimum amount of CH2Cl2 and then, precipitated by addition of ethyl ether. After filtration the resulting solid was recrystallized with 2-isopropanol (IPA). 13C NMR (67.8 MHz, CDCl3) δ143.92, 132.38, 129.1, 127.29, 67.99-70.22, 21.09.
Method B. To a soluti...
example 2
[0064]
PEG-chloride. A solution of 12KDamPEG-OH (12 g, 0.1 mmol) was azeotroped for 2 hours in toluene. This mixture was cooled to 30° C. and thionyl chloride was then added. The reaction mixture was refluxed for 18 hours, followed by partial removal of the solvent, and precipitation of the product with ethyl ether. The solid was collected by filtration, washed with ethyl ether, and recrystallized from isopropanol to yield the product (10.8 g, 0.81 mmol). 13C NMR (67.8 MHz, CDCl3) δ 69.64-70.9, 42.5.
PEG-azide (PEG-N3). To a solution of 12KDamPEG-Cl (10.6 g, 0.883 mmol) in anhydrous DMF was added NaN3 (919 mg, 35.5 mmol). The reaction was heated at 80° C. for 24 hours. After addition of ethyl ether, the solid was collected by filtration. The solid residue was dissolved in DCM and the solution was washed with water three times, dried over NaSO4, filtered and evaporated under vacuum. The resulting solid was recrystallized from DCM / ethyl ether to give the PEG-azide (9.54 g, 0.795 mmol...
example 3
mPEG 30K RNL 9 Linker
[0065] In this example, the 30KDamPEG-NH2 of Example 1 is converted into the activated PEG linker according to the following reaction scheme.
mPEG30K RNL9 OTBDMS 23:
To a solution of alcohol, 22 (238 mg, 1 mmol, 6 eq) in anhydrous CH3Cl were added DSC (235 mg, 0.92 mmol, and 5.5 eq) and pyridine (88 μL, 1.08 mmol, 6.5 eq). The resulting suspension was heated to reflux overnight, cooled to room temperature and added to a solution of 30KDamPEG-NH2 (hereinafter 21) (5 g, 0.17 mmol, 1 eq) in 25 mL of anhydrous CH3Cl. After stirring at room temperature for 3 days, the solvent was evaporated under vacuum. The resulting solid was dissolved in the minimum amount of dichloromethane and then, precipitated by addition of ether, filtered and recrystallized with CH3CN / IPA to give 4.85 g (94% yield). GPC: 98.39%. 13C NMR (75.4 MHz, CDCl3) δ 154.47, 149.59, 137.90, 126.52, 121.02, 69.09-71.65 (PEG), 64.24, 58.83, 40.83, 25.84, 18.27, 5.28.
mPEG30K RNL9OH 24:
To a solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com