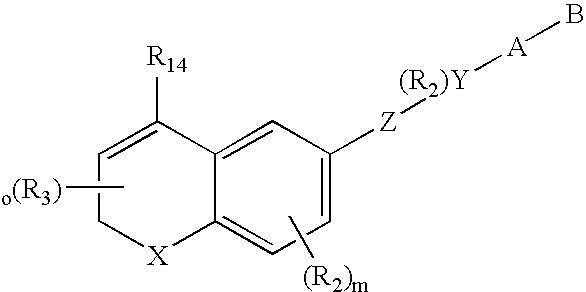
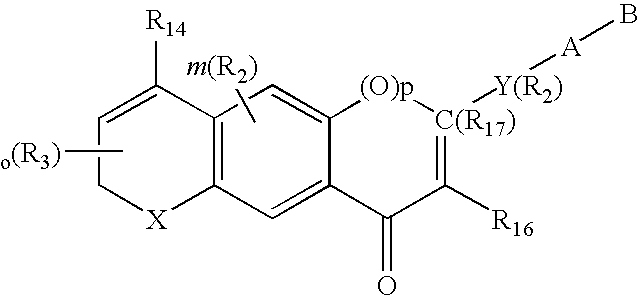
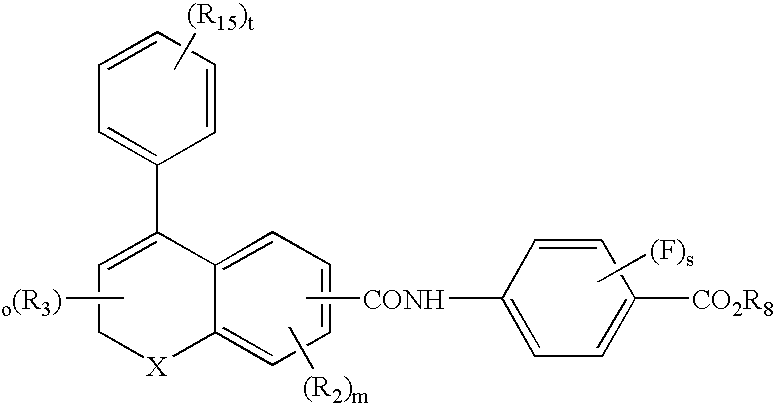
Male anti-fertility agents
a technology of anti-fertility agents and male mammals, which is applied in the direction of biocide, drug compositions, peptide/protein ingredients, etc., can solve the problems of 100% effective, health risks of continued long-term use of contraceptive hormones, and increased risk of certain forms of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Oral Treatment of Spague-Dawley Rats with AGN 194310
[0152] Ninety-eight male and ninety-eight female Sprague-Dawley (Crl:CD®(SD) IGS BR) Charles River, Hollister, Calif. 95023) rats, approximately 8 to 10 weeks old, were used for the study. The rats were divided into the following groups: non-treated control, vehicle control, 0.005 mg / kg / day, 0.015 mg / kg / day and 0.15 mg / kg / day AGN 194310. AGN 194310 has the following chemical structure:
This compound, 4-[[4-(4-ethylphenyl)-2,2-dimethyl-(2H)-thiochromen-6-yl]-ethynyl]-benzoic acid, may be synthesized using conventional organic synthetic means. The following reaction scheme is Applicants' currently preferred method of making this compound.
[0153] Step 1: A heavy-walled screw cap tube was charged with 3-methyl-2-butenoic acid (13.86 g, 138.4 mmol), 4-methoxy thiophenol (20.0 g, 138.4 mmol), and piperidine (3.45 g, 41.6 mmol). This mixture was heated to 105° C. for 32 hours, cooled to room temperature and dissolved in EtOAc (700 mL)....
example 2
Topical Treatment of Spague-Dawley Rats with AGN 194310
[0188] An experiment was conducted in a manner substantially similar to that described in Example 1, with the following differences. Twenty-nine male and twenty-nine female Sprague-Dawley rats, approximately 7 weeks old were used for the study. Five rats / sex / group were designated as Main Study animals: (vehicle control, 0.025 mg / kg / day AGN 194310, and 0.25 mg / kg / day AGN 194310), and 7 / sex / group designated as toxicokinetic satellite animals (0.025 mg / kg / day AGN 194310 and 0.25 mg / kg / day AGN 194310). No “vehicle alone” control group was made for the toxicokinetic satellite animals. In this study there was no Recovery group.
[0189] The animals' backs were maintained shaven during the course of the study for application of the topical cream. The animals were treated daily with a topical formulation containing either AGN 194310 vehicle cream alone, 0.01% (w / w) AGN 194310 in the same vehicle cream, or 0.1% (w / w) AGN 194310 in the sam...
example 3
Reversibility of Spermatogenic Arrest
[0195] This experiment was conducted in a manner substantially similar to that of Example 1. Groups of male Sprague Dawley rats were treated orally for 4 weeks with either 0, 0.075, or 0.150 mg / kg / day of AGN 194310. Three to six animals from each group were sacrificed after 2 weeks of treatment, 6 animals from each group were sacrificed following 4 weeks of treatment and 6 animals from each group were sacrificed after 18-23 weeks of subsequent recovery after cessation of treatment. Histological and pathological examinations were done of the sacrificed animals, as in Example 1. Additionally, the animals in the 23 week recovery group were mated to normal, untreated female Sprague Dawley rats before being sacrificed to assess the reproductive function.
[0196] As in the previous examples, the control group of rats (no drug) displayed no abnormal histological or biochemical differences during the time course of the experiment, except for a single ind...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com