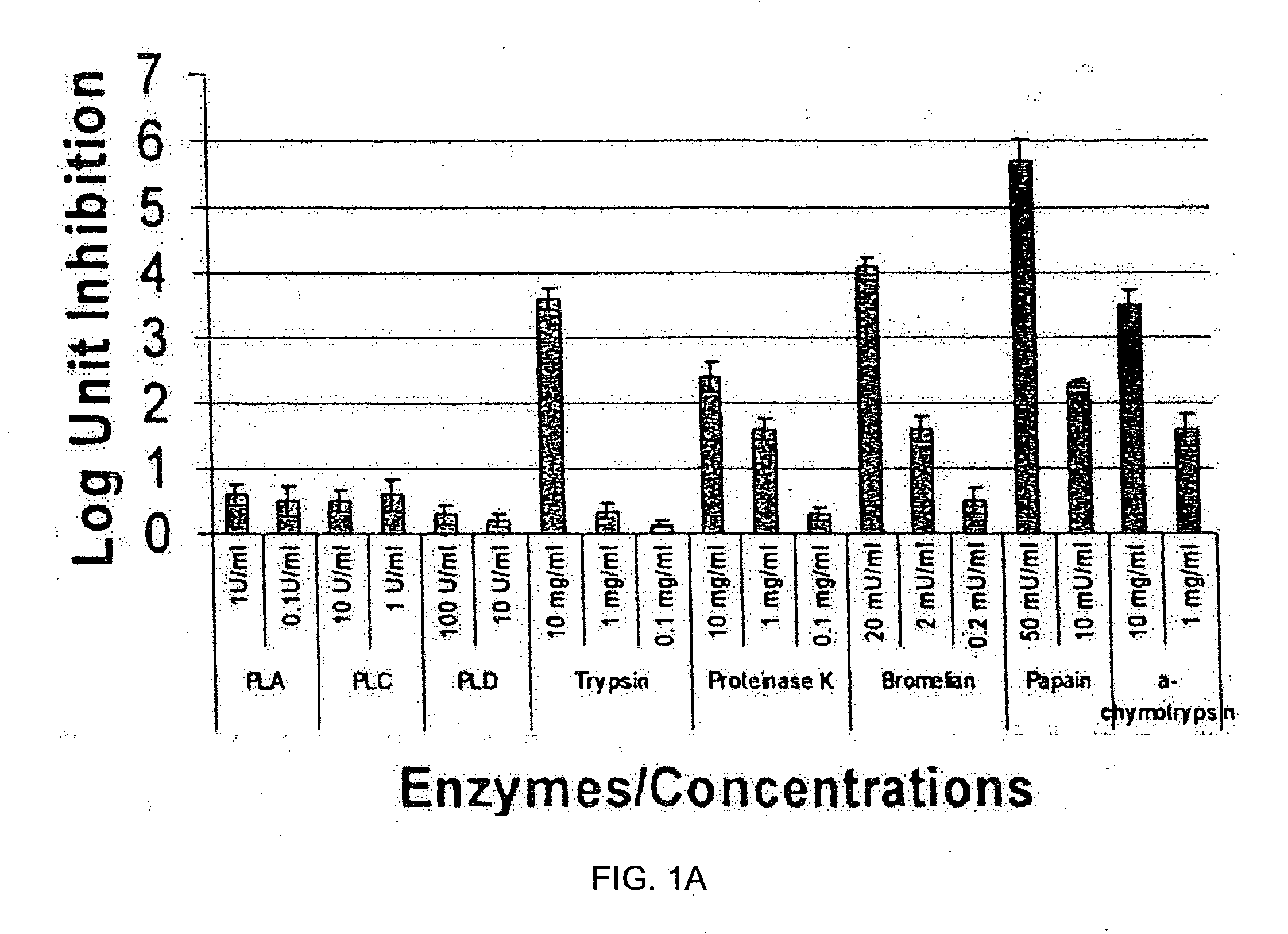
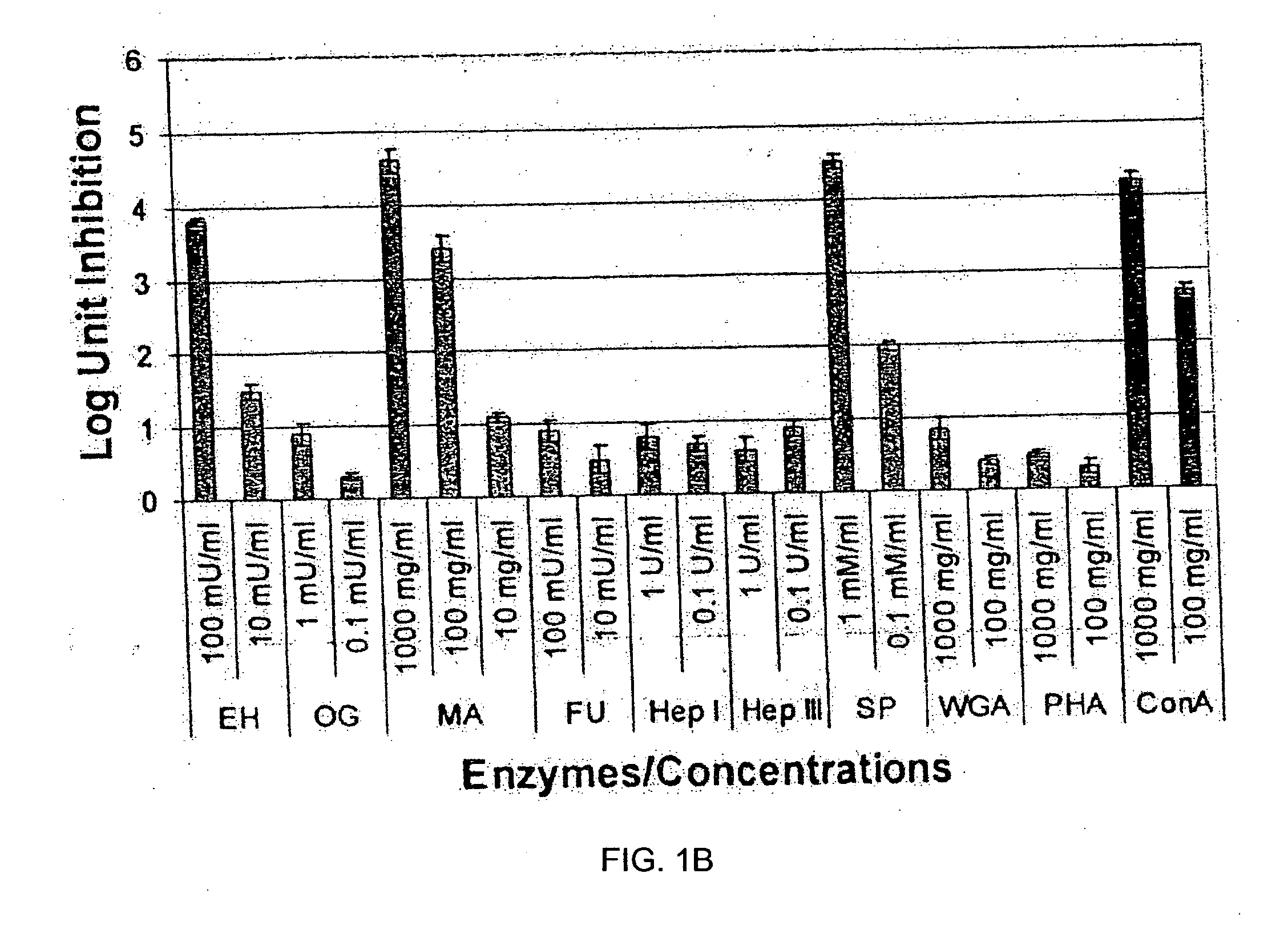
In ovo delivery of an immunogen containing implant
an immunogen and implant technology, applied in the field of in ovo delivery of an immunogen containing implant, can solve the problems of significantly affecting the effectiveness of a vaccination program, inability to use injectable vaccines or immunize birds, and inability to reduce stress, reduce internal variability of response to immunization in a flock, and increase the overall vaccine efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
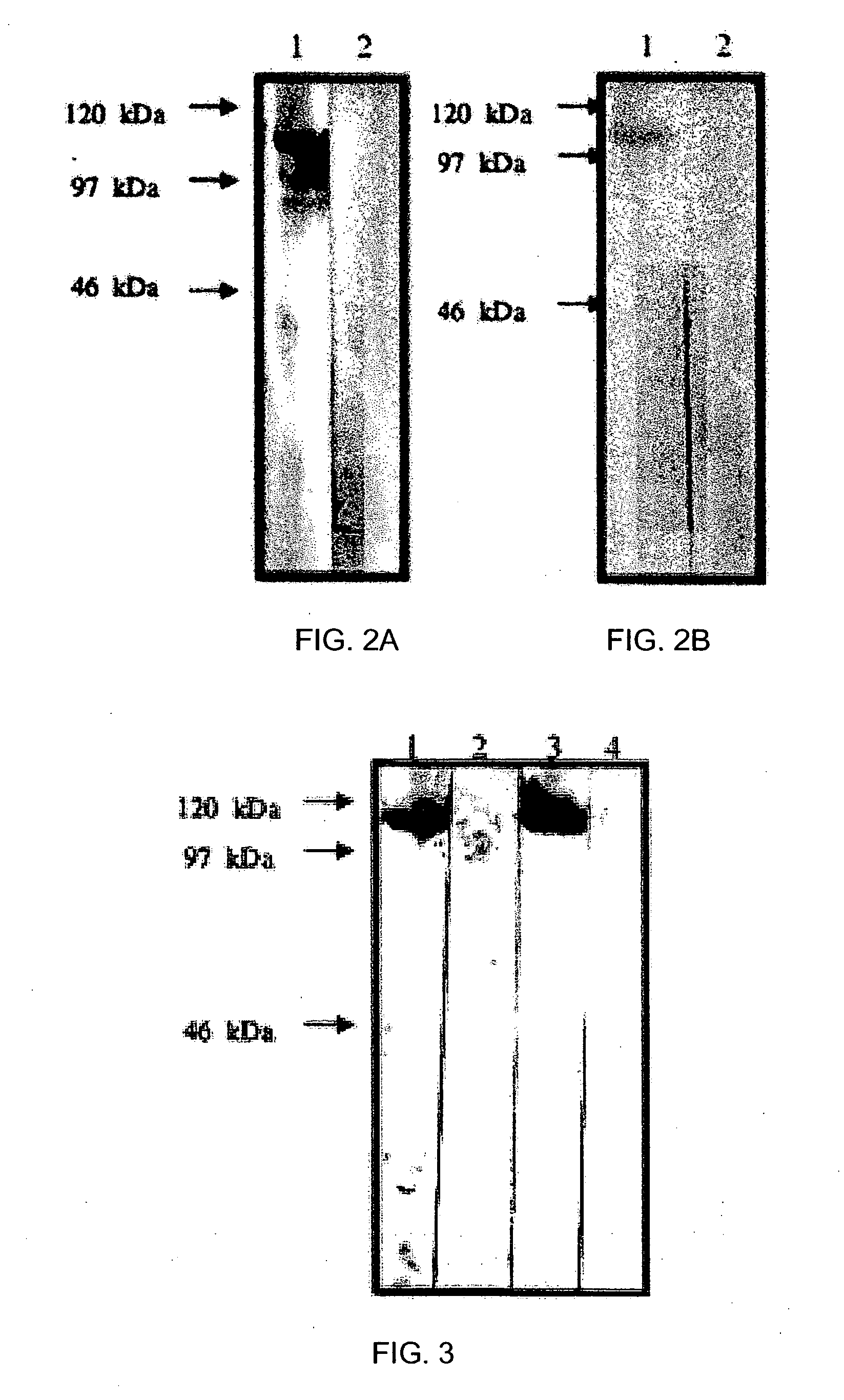
Image
Examples
example 1
Preparation of Combined Sustained / Delayed Release Microsphere Implants
[0086] 3 g of medium viscosity alginic acid (Keltone HV, Monsanto Chemical Co.), was dissolved into 100 ml of 0.04 M sodium phosphate pH 5.7. The resulting suspension was blended at 3000 rpm for 10 minutes using a Dyna-mix stirrer with a 18 inch stainless steel impeller (Fisher Scientific, Pittsburgh, Pa.) until a homogenous solution was formed. Xanthan gum (1.5%) (Xanthural 11K, Monsanto Chemical Co.) was prepared in 0.04 M sodium phosphate and added to the alginic acid to give a final xanthan gum concentration of 0.023%. The xanthan gum and alginic acid solution was then mixed thoroughly and sterilized by autoclaving for 15 min at 121° C. The final viscosity of the mixture at 25° C. was approximately 3,500 cps. The solution was stored at 25° C. until it was used.
[0087] Spherical cellulose, dextran or agarose beads having a particle diameter of 50-150 μm and functionalized with diethylaminoethyl (DEAE) to give ...
example 2
Evaluation of Antigen Release from Microsphere Implants in Poults
[0090] Microspheres were prepared as described in Example 1 and including the SRP-Porin antigens of Pasteurella multocida P-1059. The SRP Porin antigen suspension was adjusted to three different concentration, 250 μg, 500 μg and 1000 μg per bird dose. A placebo was prepared containing all ingredients except the SRP-Porin antigen and was used as the control dose.
[0091] 200 forty-day-old turkey poults (hybrid hens) were obtained from Willmar Poultry Company's Commercial hatchery, (Willmar, Minn.) and equally divided into four groups, designated as I-IV. All birds received a 0.5 cc subcutaneous injection of the appropriate vaccine implant in the lower neck region (group I-controls, group II-250 μg; group III-500 μg and group IV-1000 μg). Birds were colored to maintain identity of treatment groups and randomly distributed between two isolation rooms.
[0092] At 7 day intervals through eight weeks of age, four birds / group ...
example 3
Vaccination of Poultry with Siderophore Receptor Proteins-Porins Incorporated in Alginic Acid Microspheres
[0095] Microspheres were prepared as described in Example 1 containing the SRP-Porin antigens of Escherichia coli. A placebo was prepared containing all ingredients of the microspheres except the SRP-porin antigen and was used for the non-vaccinated control group.
[0096] Twelve hundred-day-old turkey poults (hybrid hens) were obtained from Willmar Poultry Company's commercial hatchery (Willmar, Minn.) and equally divided into three Groups designated as A, B and C. All birds in Groups A and C received a 0.5 cc subcutaneous injection of microspheres. Group A received the placebo and remained as non-vaccinated, implanted controls. Group C received 0.5 cc of the SRP-porin containing microspheres at a bird dose of 941 μg. Group B received no microspheres or other vaccine and remained as non-vaccinated, non-implanted birds.
[0097] Each Group (A, B, C) of birds were colored at the hat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com