Sintered body of titanium compound
a titanium compound and sintered body technology, applied in the field of titanium compounds, can solve the problems of low affinity with living tissues, low reactivity of titanium to living bodies, and difficulty in combining titanium with bone tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Titanium Compound:
[0062] 0.1 mol of calcium nitrate (Ca(NO3)2) and 0.1 mol of titanium suflate (TiSO4) were dissolved in about 500 ml of water, followed by neutralizing with an alkali. After adding 0.06 mol of phosphoric acid (H3PO4), aqueous ammonia was added to adjust pH to 9.0, followed by stirring at 100° C. for 6 hours. The precipitates obtained was filtered off, and dried, thereby obtaining about 10 g of a powder of the titanium compound represented by the formula (1) or (2).
example 2
Sintering of Titanium Compound:
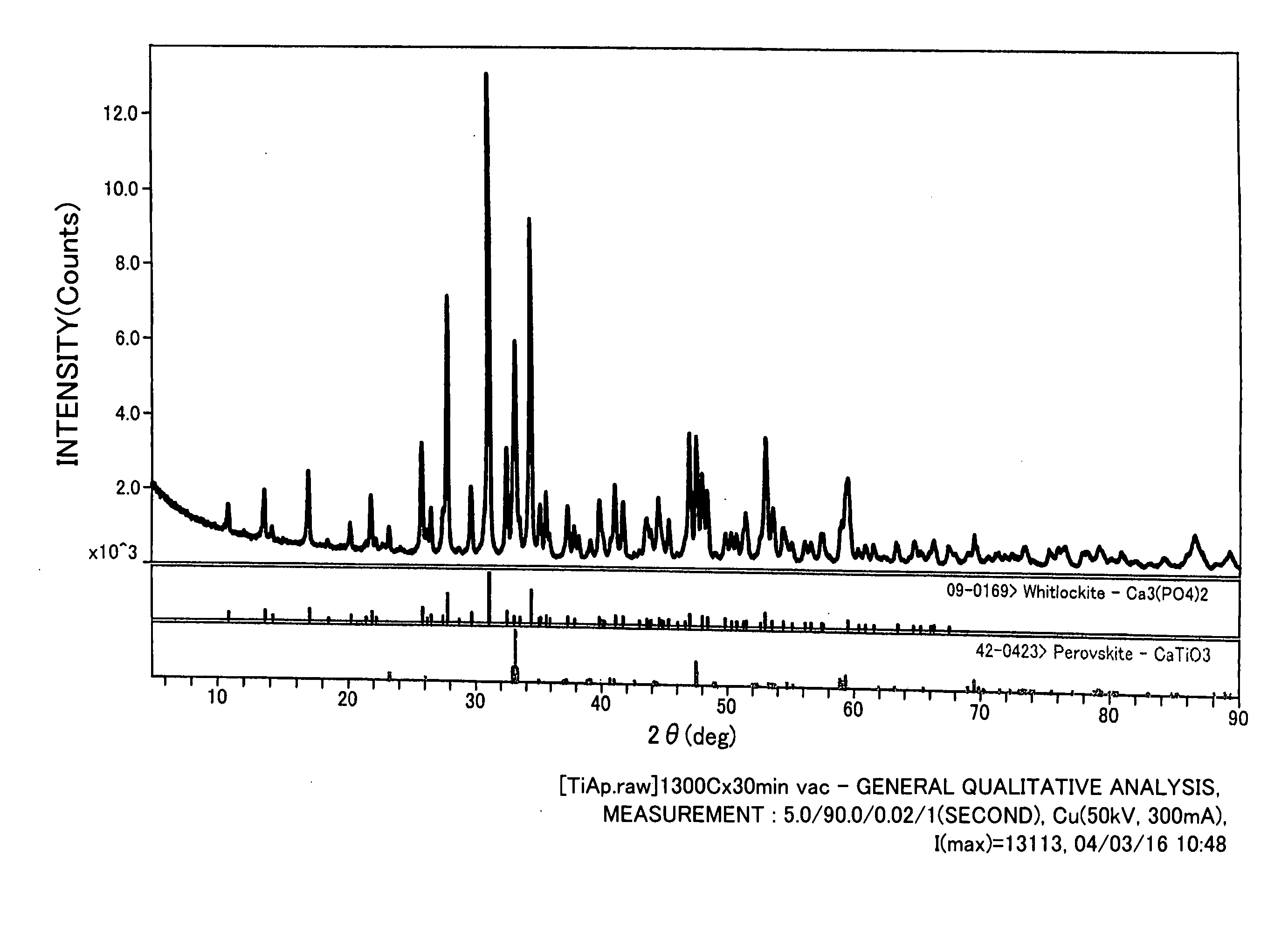
[0063] About 3 g of the powder of the titanium compound obtained in Example 1 was kneaded with purified water, and placed in a mold, molded, and then air-dried. The air-dried product was dried in a drying oven at 100° C. for 24 hours. The dried sample was placed in a vacuum heat-treating machine, and held at various temperatures under an atmospheric pressure or in vacuum (10−4 Pa) for 30 minutes to sinter the same. After stopping the heating, the sample was allowed to stand to room temperature. Regarding the sample sintered in vacuum, it was allowed to stand to room temperature, and after introducing argon gas, and was taken out. Regarding the sintered body of the titanium compound obtained, crystal analysis by X ray diffraction was conducted. FIG. 1 shows the result of X ray diffraction in the case of sintering at 1,300° C. in vacuum. Further, FIG. 1 also shows X ray diffraction patterns of perovskite and whitlockite. Further, the results are summar...
example 3
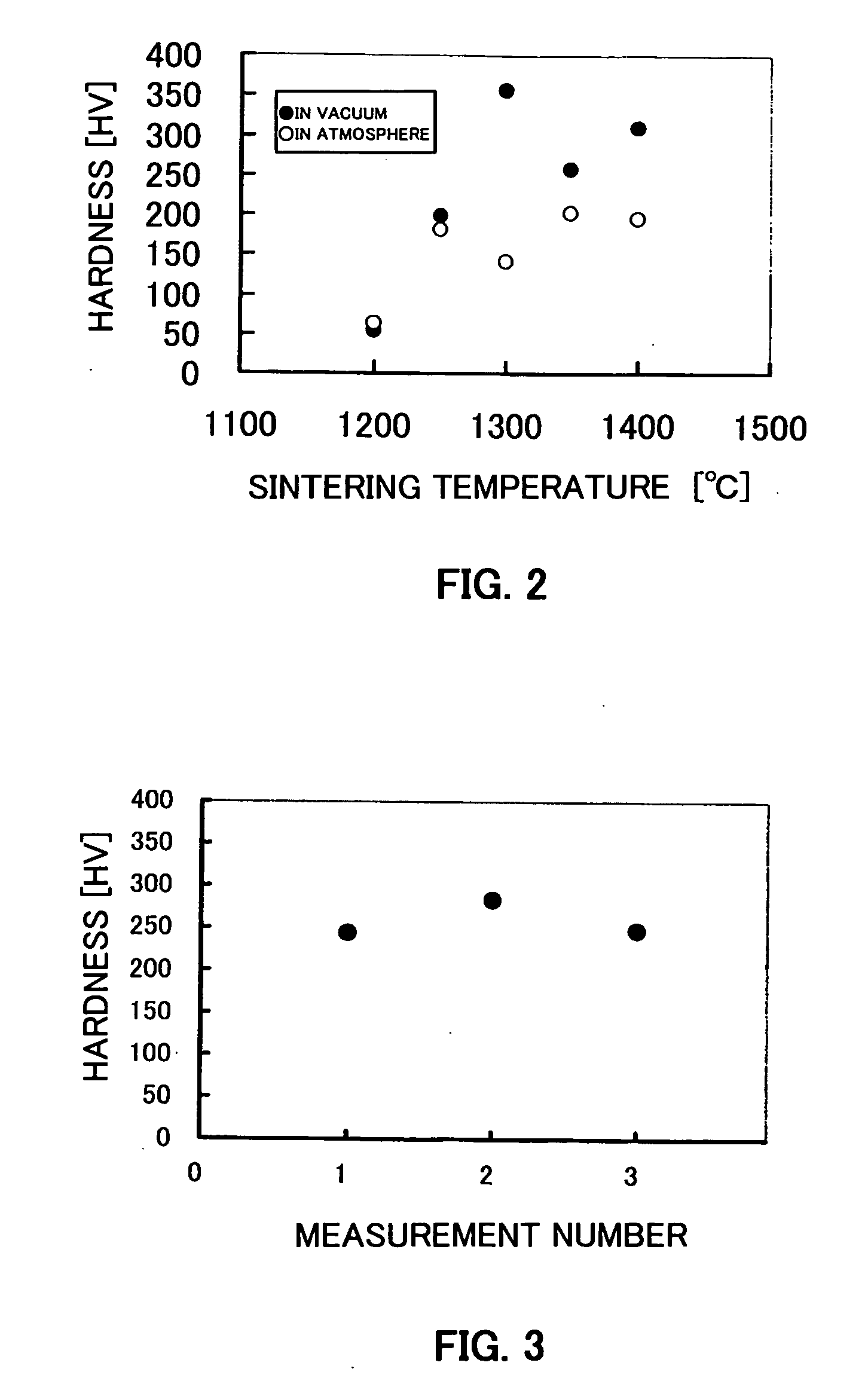
[0066] About 3 g of a powder of a titanium compound produced by a coprecipitation method was kneaded with purified water, placed in a mold, molded, and then air-dried. The air-dried product was dried in a drying oven at 100° C. for 24 hours. The dried sample was placed in a vacuum heat-treating machine, and held at various temperatures under an atmospheric pressure or in vacuum (10−4 Pa) for 15 minutes to sinter the same. After stopping the heating, the sample was allowed to stand to room temperature. Regarding the sample sintered in vacuum, it was allowed to stand to room temperature, and after introducing argon gas, and was taken out. Regarding the obtained sintered body of the titanium compound, microvickers hardness was measured. The results are shown in FIG. 2.
[0067] It is seen from FIG. 2 that a sintered body of a titanium compound, having high hardness is obtained. Further, it is seen that a sintered body having higher hardness can be obtained in the case of sintering in vac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com