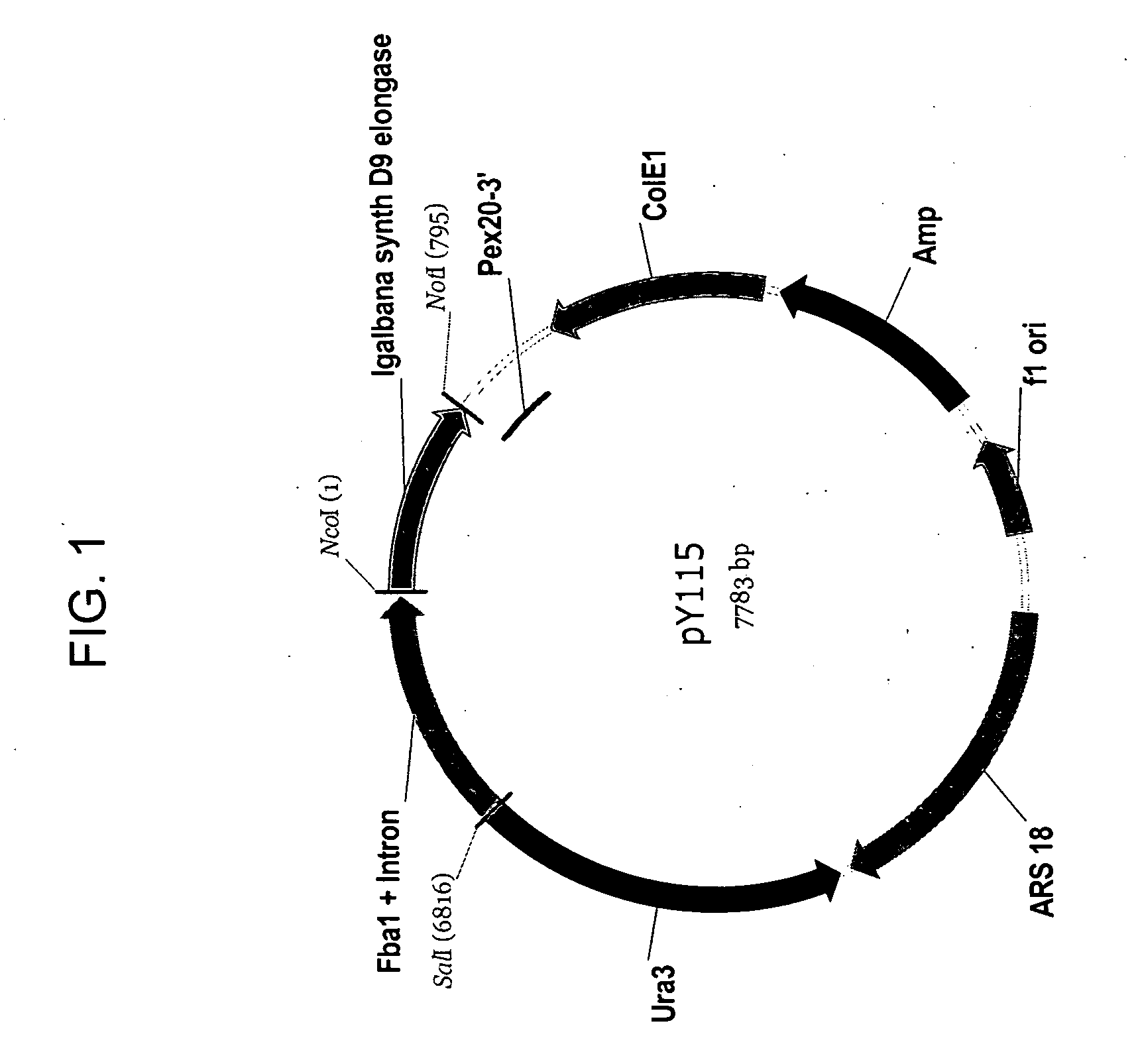
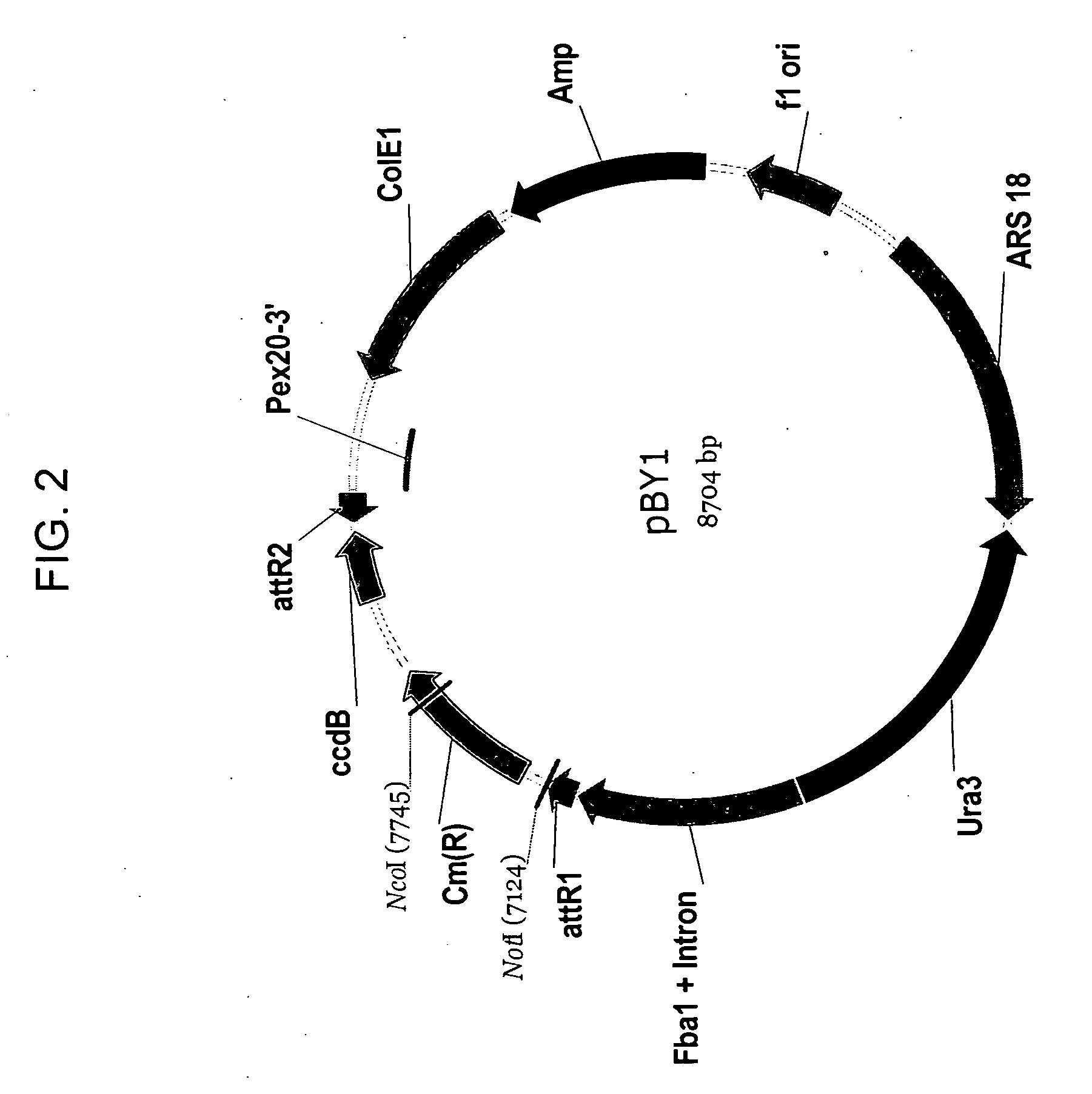
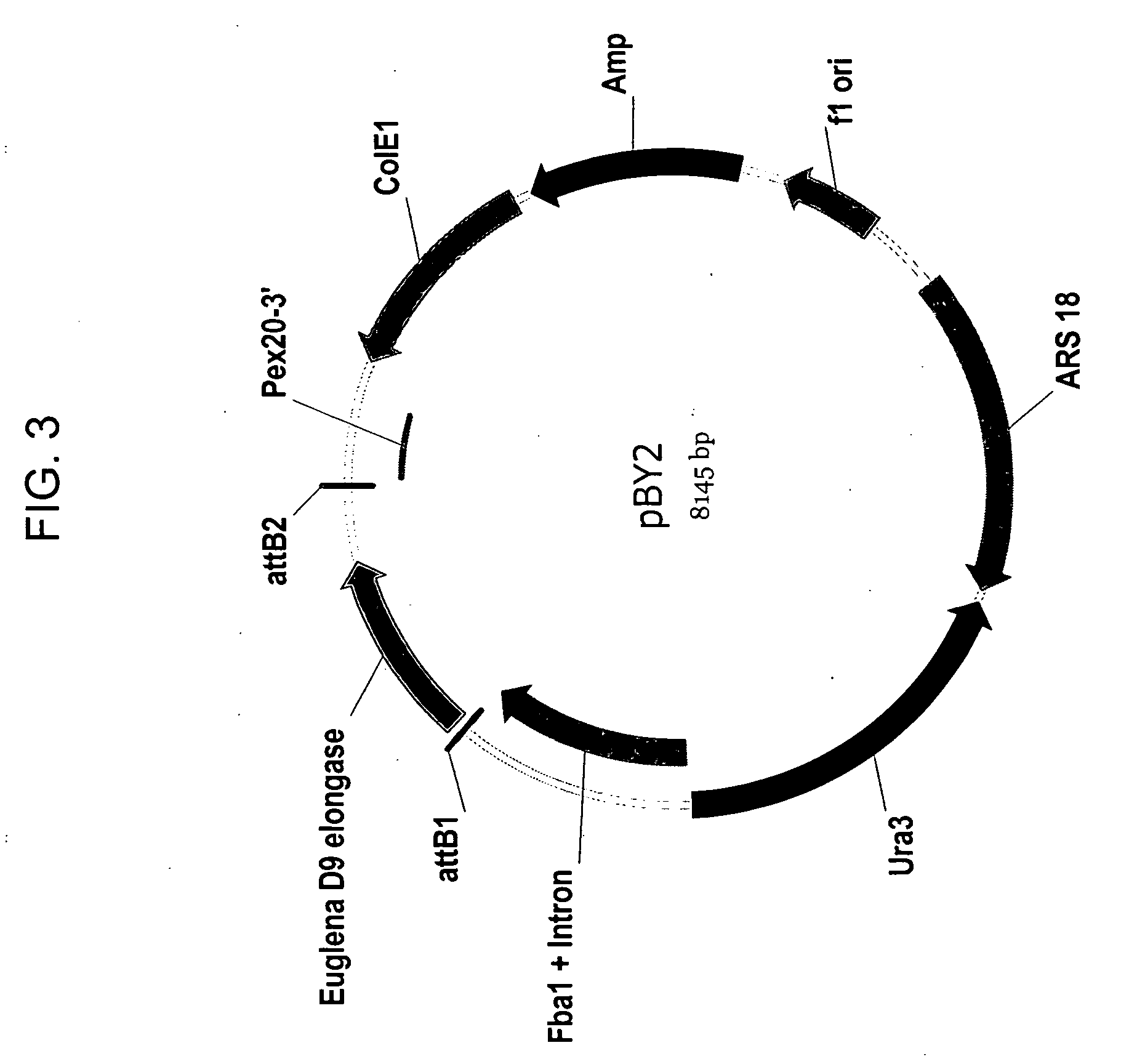
Delta-9 elongases and their use in making polyunsaturated fatty acids
a technology of delta9 and elongases, which is applied in the field of polyunsaturated fatty acids encoding delta9 elongases, can solve the problems of insufficient commercial needs, insufficient study of pufas from natural sources and chemical synthesis, and inability to efficiently synthesize by the human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Euglena gracilis Growth Conditions, Lipid Profile and mRNA Isolation
[0410]Euglena gracilis was obtained from Dr. Richard Triemer's lab at Michigan State University (East Lansing, Mich.). From 10 mL of actively growing culture, a 1 mL aliquot was transferred into 250 mL of Euglena gracilis (Eg) Medium in a 500 mL glass bottle. Eg medium was made by combining 1 g of sodium acetate, 1 g of beef extract (U126-01, Difco Laboratories, Detroit, Mich.), 2 g of Bacto® tryptone (0123-17-3, Difco Laboratories), 2 g of Bacto®) yeast extract (0127-17-9, Difco Laboratories) in 970 mL of water. After filter sterilizing, 30 mL of soil-water supernatant (15-3790, Carolina Biological Supply Company, Burlington, N.C.,) was aseptically added to give the final Eg medium. Euglena gracilis cultures were grown at 23° C. with a 16 h light, 8 h dark cycle for 2 weeks with no agitation.
[0411] After 2 weeks, 10 mL of culture was removed for lipid analysis and centrifuged at 1,800×g for 5 min. The pellet was ...
example 2
Euglena gracilis cDNA Synthesis, Library Construction and Sequencing
[0413] A cDNA library was generated using the Cloneminer™ CDNA Library Construction Kit (Cat. No. 18249-029, Invitrogen Corporation, Carlsbad, Calif.) and following the manufacturer's protocol provided (Version B, 25-0608). Using the non-radiolabeling method, cDNA was synthesized from 3.2 μg of mRNA (described above) using the Biotin-attB2-Oligo(dT) primer. After synthesis of the first and second strand, the attb1 adapter was added, ligated and the cDNA was size fractionated using column chromatography. DNA from fractions 7 and 8 (size ranging from ˜800-1500 bp) were concentrated, recombined into PDONR T222 and transformed into E. coli ElectroMAX™ DH10B™ T1 Phage-Resistant cells (Invitrogen Corporation). The Euglena gracilis library was named eeg1 c.
[0414] For sequencing, clones first were recovered from archived glycerol cultures grown / frozen in 384-well freezing media plates, and replicated with a sterile 384 pi...
example 3
Identification of Long-Chain Polyunsaturated Fatty Acid Elongation Enzyme Homologs from Euglena gracilis cDNA Library eeg1c
[0416] cDNA clones encoding long-chain polyunsaturated fatty acid elongation enzyme homologs (LC-PUFA ELO homologs or delta-9 elongases) were identified by conducting BLAST (Basic Local Alignment Search Tool; Altschul et al., J. Mol. Biol. 215:403-410 (1993)) searches for similarity to sequences contained in the BLAST “nr” database (comprising all non-redundant GenBank CDS translations, sequences derived from the 3-dimensional structure Brookhaven Protein Data Bank, the last major release of the SWISS-PROT protein sequence database, EMBL, and DDBJ databases). The cDNA sequences obtained in Example 2 were analyzed for similarity to all publicly available DNA sequences contained in the “nr” database using the BLASTN algorithm provided by the National Center for Biotechnology Information (NCBI). The DNA sequences were translated in all reading frames and compared ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com