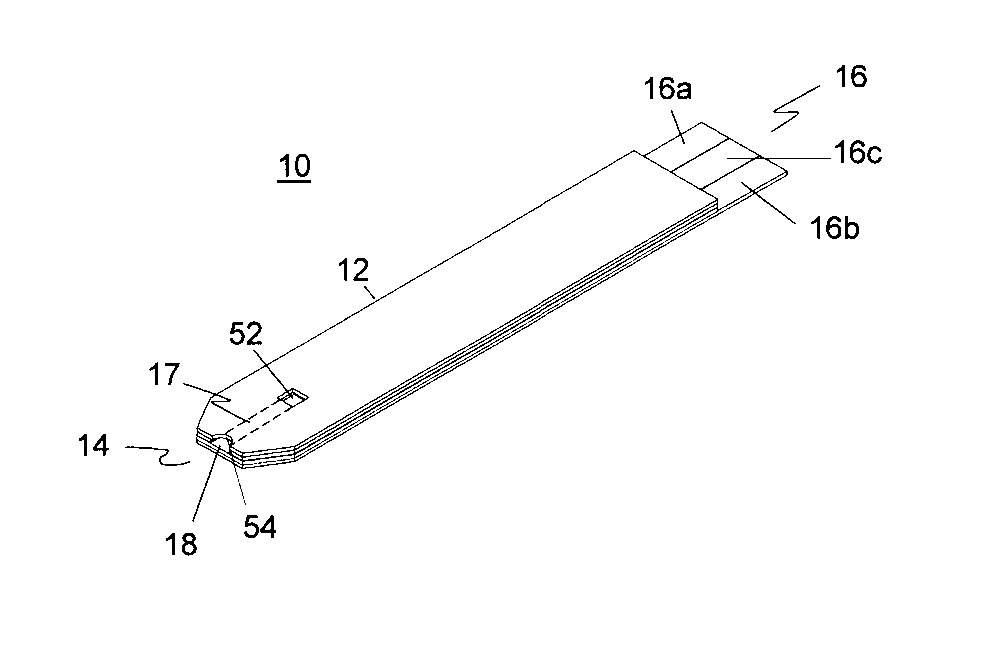
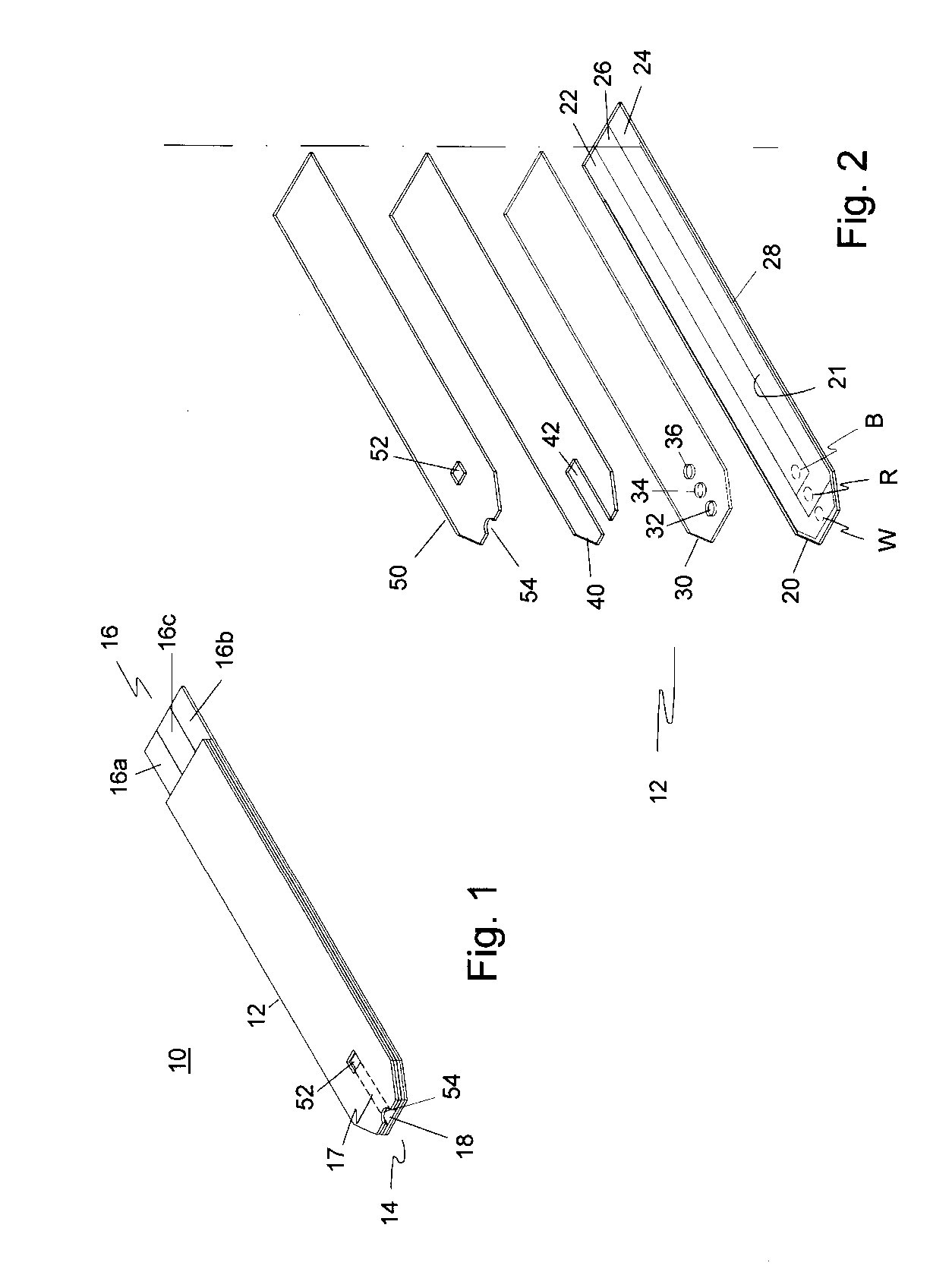
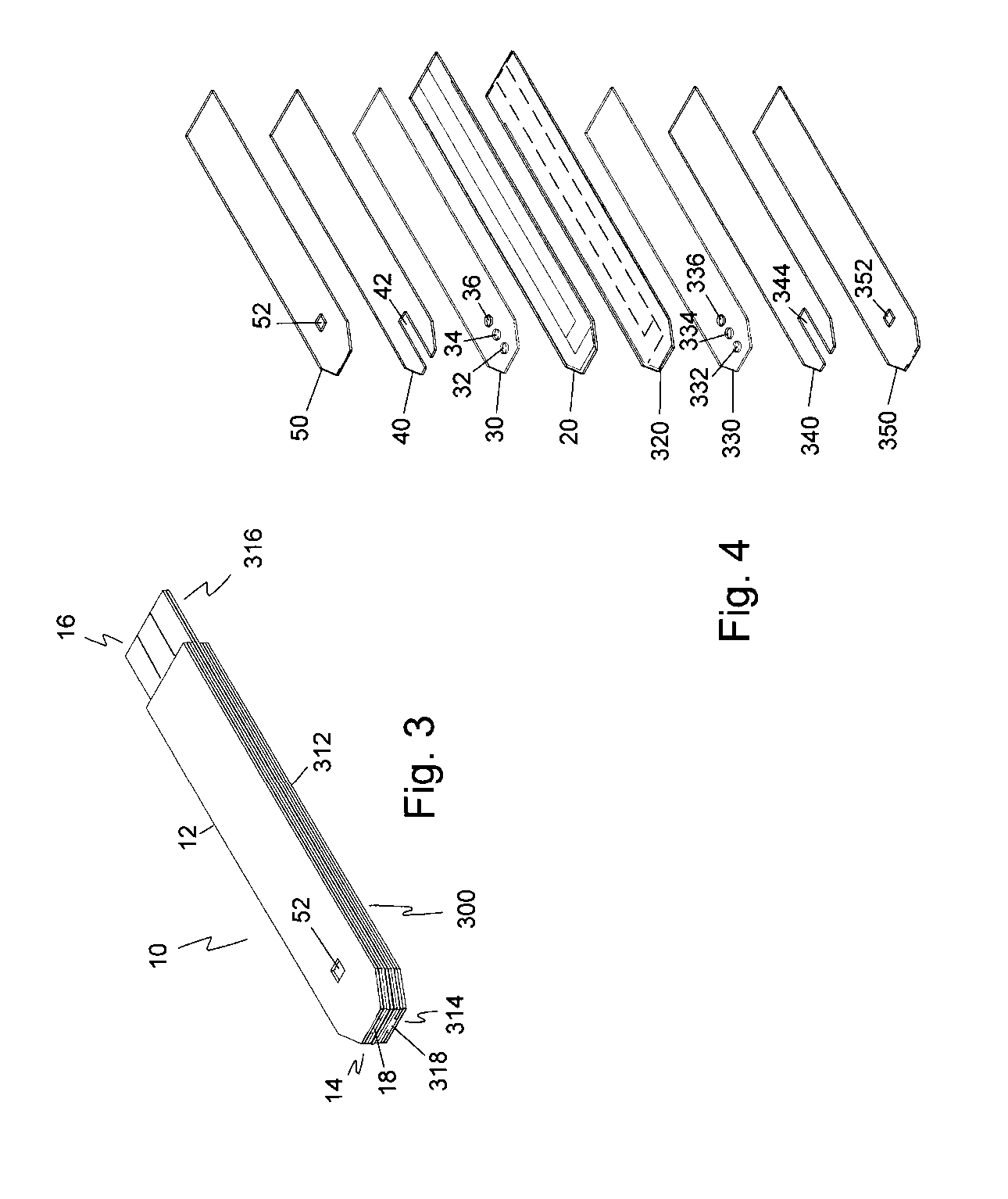
Disposable urea sensor and system for determining creatinine and urea nitrogen-to-creatinine ratio in a single device
a technology of urea nitrogen and creatinine, which is applied in the field of disposable electrochemical sensors, can solve the problems of high urea concentration in blood, malfunctioning kidneys will have difficulty in getting rid of excess urea, and the body is toxic to the extent of high urea concentration, and achieves the effect of fast response tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0138] Demonstration of Determination of Urea Concentration with Ag-AgCl Reference
[0139] Blood samples with different urea concentrations were tested with the urea sensors of the present invention having a silver-silver chloride reference electrode and with the Vitros® Chemistry System DT6011 (Ortho-Clinical Diagnostics, Inc., Rochester, N.Y.). FIG. 14 shows the determination of BUN concentration in mg / dL in blood samples using the urea sensors of the present invention to varying urea concentrations in the blood samples. It is noted that these determinations are raw data before any compensation for hematocrit, in order to show the actual correlation of the unmanipulated data.
[0140] As seen from the graph, the sensors of the present invention respond to the urea concentration in the blood samples over a tested range of about 17 mg / dL to about 139 mg / dL of blood urea nitrogen. The average coefficient of variation for the sensors of the present invention using a silver / silver-chlorid...
example 2
[0141] Demonstration of Determination of Urea Concentration with Redox Reference
[0142] Blood samples with different urea concentrations were tested with the urea sensors of the present invention having a redox mediator / couple reference matrix and with a Dimension® Chemistry System (Dimension RxL, Dade Behring, Inc.). FIG. 15 shows the determination of BUN concentration in mg / dL in blood samples using the urea sensors of the present invention to varying urea concentrations in the blood samples.
[0143] As seen from the graph, the sensors of the present invention respond to the urea concentration in the blood samples over a tested range of about 19 mg / dL to about 187 mg / dL of blood urea nitrogen. The average coefficient of variation for the sensors of the present invention having a redox mediator / couple based reference is about 6.4%.
[0144] Note that the testing results can be corrected for hematocrit interference. The hematocrit of the blood sample can be calculated based on the impe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com