Electrolysis cell for synthesizing perchloric acid compound and method for electrolytically synthesizing perchloric acid compound
a perchloric acid compound and electrolysis cell technology, applied in the direction of electrodes, electrolysis processes, electrolysis components, etc., can solve the problems of deterioration, unstable electrode performance, consumption acceleration, etc., and achieve high purity, easy separation of chlorine gas, the effect of high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
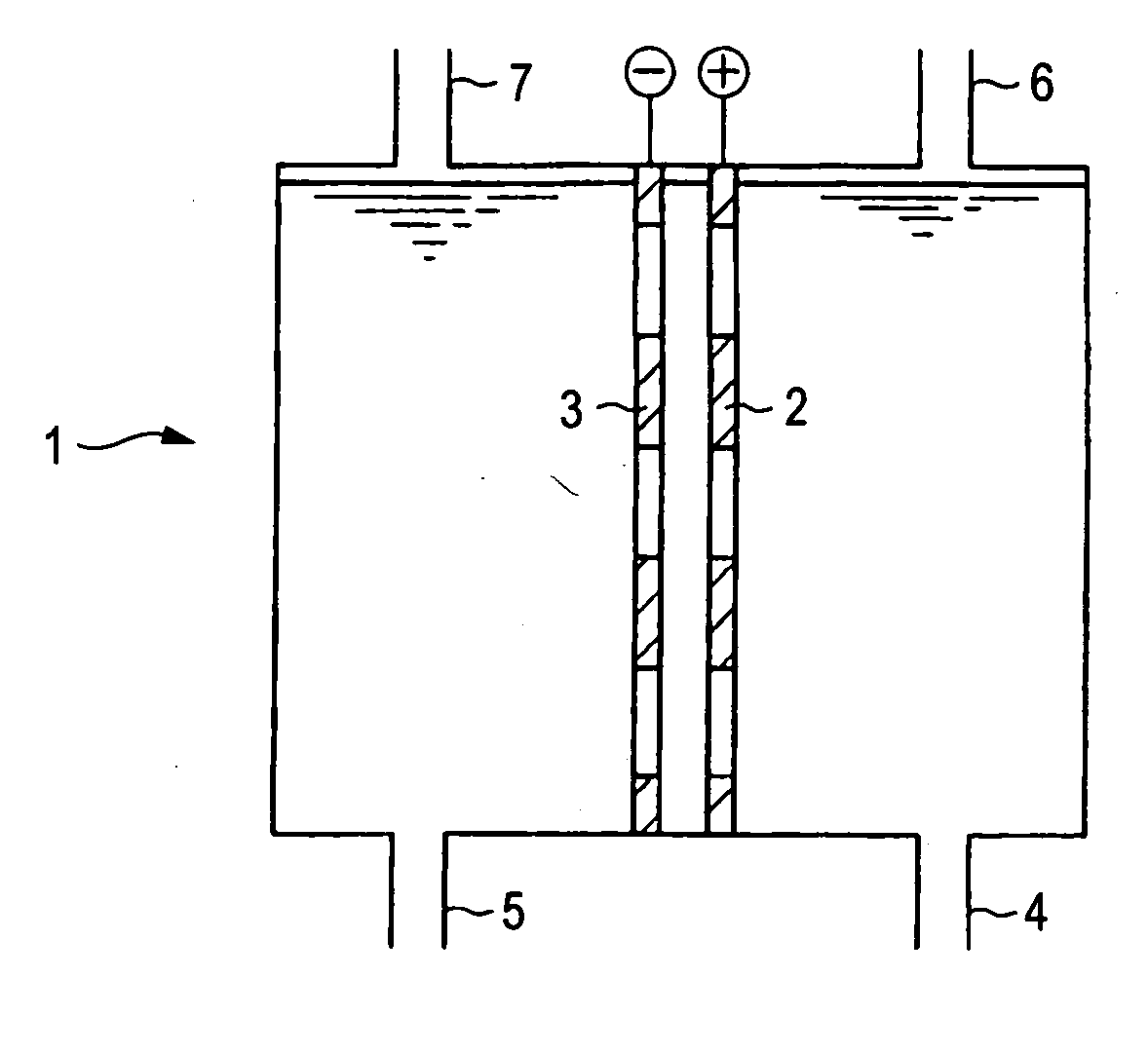
[0074] An anode obtained by forming a thickness of 5 μm of an electroconductive diamond doped with boron of 5,000 ppm on electroconductive silicon having a thickness of 1 mm serving as a substrate was used. As a cathode, a platinum-plated titanium plate having a thickness of 1 mm was used. An electrode area of each of the anode and the cathode was 20 cm2.
[0075] These electrodes were disposed in the membraneless cell (capacity: 1000 ml) shown in FIG. 1 in such a fashion that they are opposed to each other with a gap of 1 cm being defined therebetween. The cell was charged with 500 ml of a 0.02 M sodium chlorate solution, and electrolysis was performed for one hour with a current of 1 A (current density: 0.05 A / cm2) with stirring with a water temperature being kept to 35° C.
[0076] Analysis was performed by using an ion chromatography device, and a current efficiency of the perchloric acid ion generation was calculated from the concentration. Sodium hypochlorite was measured by KI ti...
example 2
[0080] An anode obtained by forming an electroconductive diamond doped with boron of 5,000 ppm on a substrate which was a niobium mesh having an original plate thickness of 2 mm was used. As a cathode, a platinum-plated titanium mesh having an original plate thickness of 1 mm was used. An electrode area of each of the anode and the cathode was 20 cm2, and a two-chamber cell wherein an ion exchange membrane (117 Nafion) was sandwiched between the anode and the cathode of the membraneless cell of FIG. 1 is assembled (gap between electrodes: 1 mm), followed by injecting 200 ml of a 0.5 M hydrochloric acid solution to the anode chamber and the cathode chamber. By performing electrolysis for one hour under conditions of 40° C. and 20 A (current density: 1 A / cm2), perchloric acid ions were obtained at a current efficiency of 12%. A chloric acid concentration during the electrolysis was lowered to 0.05 M. A cell voltage was 5.5 V A residual chlorine gas was removed by degassing.
example 3
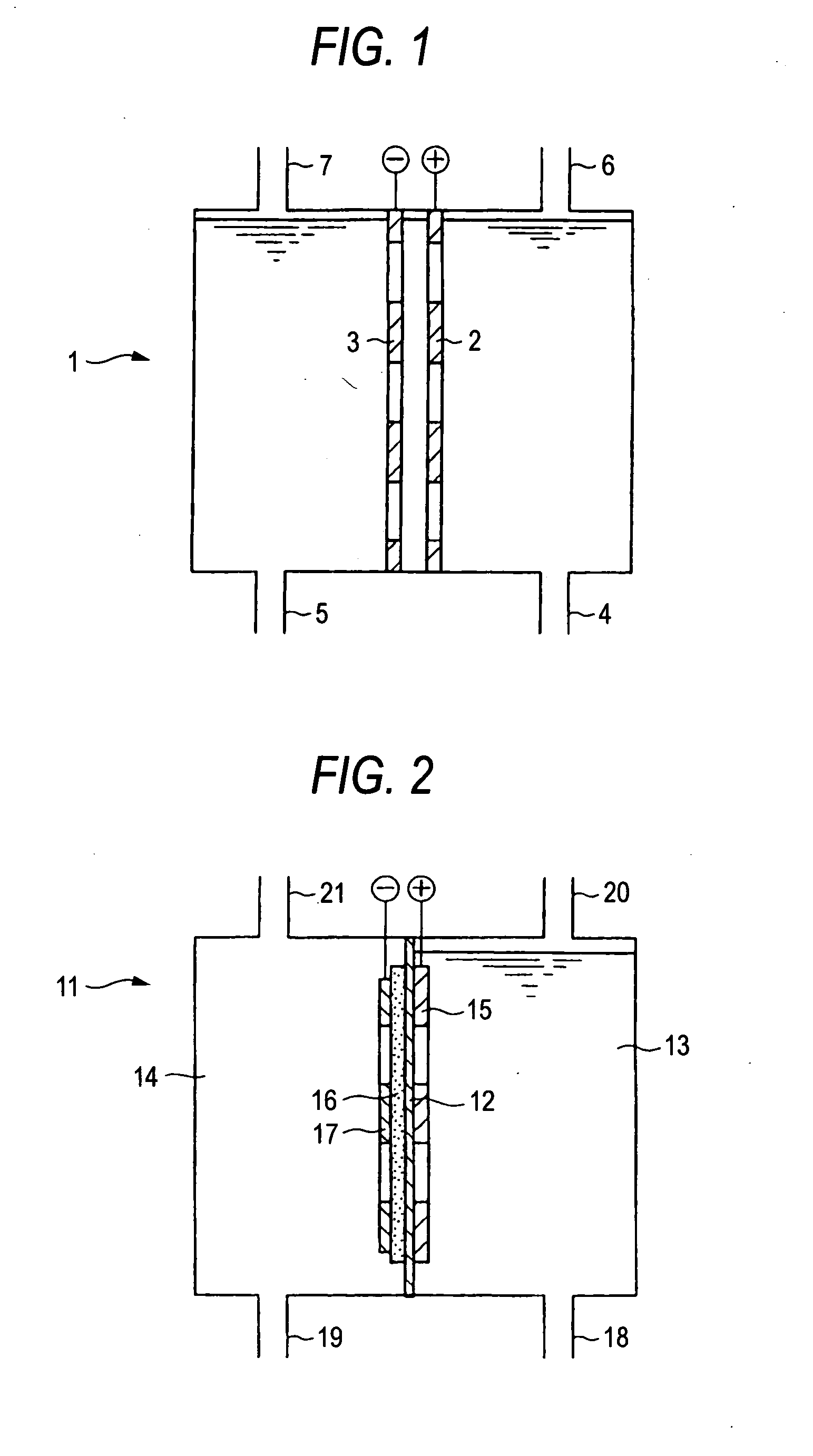
[0082] The anode of Example 2 was used, and a sheet having the size of 2 cm×2 cm×0.5 mm of thickness obtained by: mixing a graphite powder (product of Tokai Carbon Co., Ltd.; TGP-2), which was used as a catalyst, with a PTFE resin; applying the mixture on a core material which was a carbon cloth (product of Zoltek Corporation; PWB-3), and baking at 330° C. was used as the cathode. A platinum-plated titanium mesh was used as a cathode power feeding support, and a two-chamber cell wherein the ion exchange membrane of FIG. 2 (Nafion 117) was disposed between the chambers was assembled. As a result of electrolysis for one hour at 60° C. and 20 A in the same manner as in Example 2 with oxygen being supplied to the cathode chamber, perchloric acid ions were obtained at a current efficiency of 10%. A hydrochloric acid concentration during the electrolysis was lowered to 0.05 M. A cell voltage was 4.7 V.
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com