Vectors and viral vectors, and packaging cell lines for propagating same
a technology of vectors and viral cells, applied in the field of recombinant nucleic acid technology, can solve the problems of low yield of virus vectors or inefficient expression of exogenous genes in target cells, limited human gene transfer use, and high instability of newly transcribed mrna, etc., to achieve the effect of reducing the number of mrna and limiting the use of human gene transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reconstitution of a Cis Effect that Resulted from the Inactivation of the U3 Promoter / Enhancer in a Heterologous Vector (Retrovirus Vector) by the use of Irrelevant Sequence Replacement
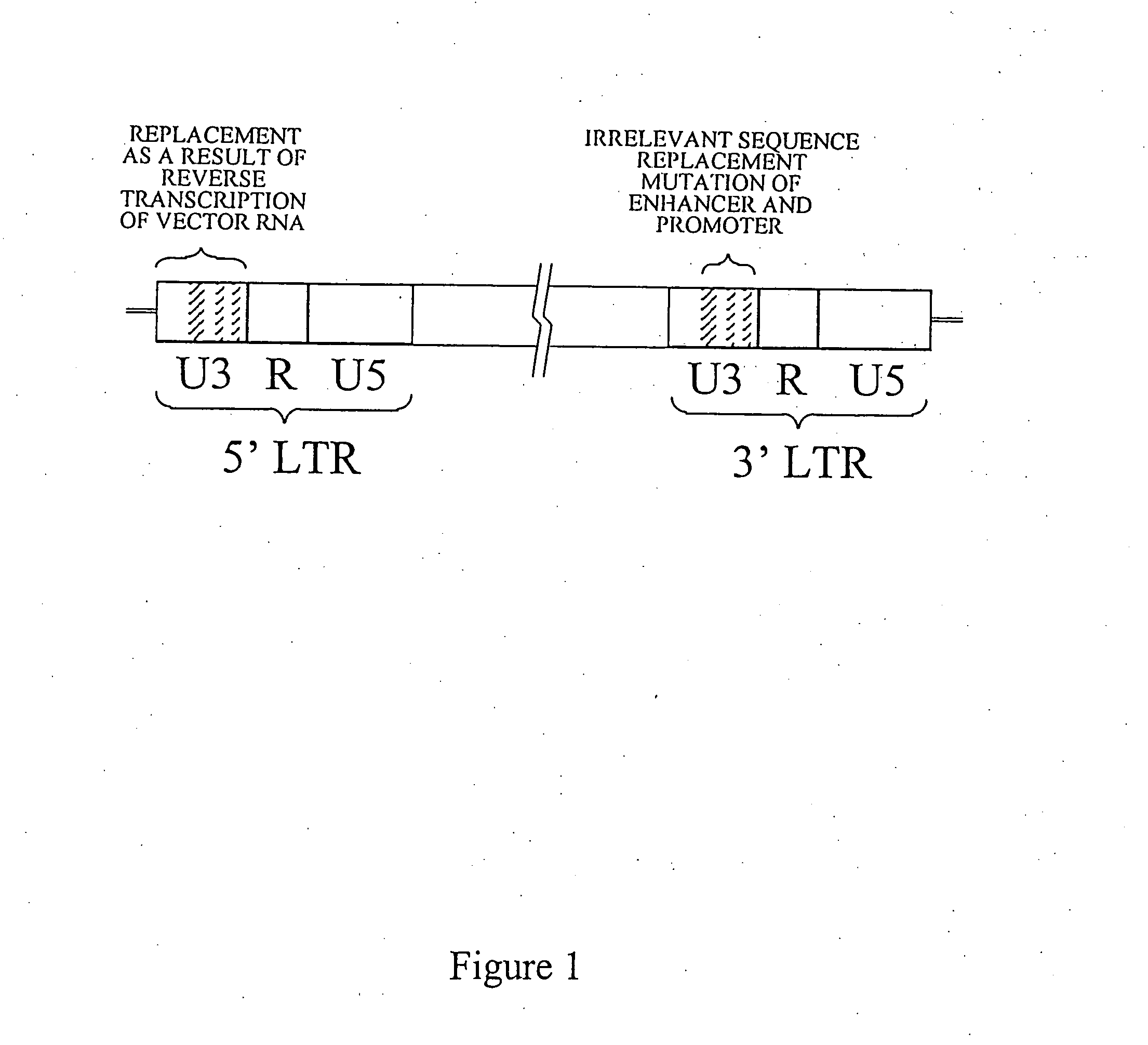
[0143] A Heterologous Vector (retrovirus vector) was prepared in which the LTR promoter and enhancer were inactivated wherein polyadenylation function was reconstituted. Replacements were made to a retroviral vector sequence (FIG. 1) present in a plasmid (pENZ1). A 188 base pair DNA fragment in the 3′ LTR U3 enhancer was replaced by 188 base pairs derived from the bacterial Neo gene (neomycin phosphotransferase) sequence through PCR strategy. Two regions of the promoter, one of 2 base pairs and one of 6 base pairs, were each replaced by restriction enzyme recognition sequences of the same size through oligonucleotide-mediated site directed mutagenesis. The removed native sequences and the non-native replacement sequences are shown in FIG. 2.
[0144] The replacements of the enhancer and promoter region...
example 2
A Retroviral Vector with Inactivated Promoter / Enhancer Which Contains a Non-Native Polyadenylation Signal (the Mouse Histone H2A614 Gene).
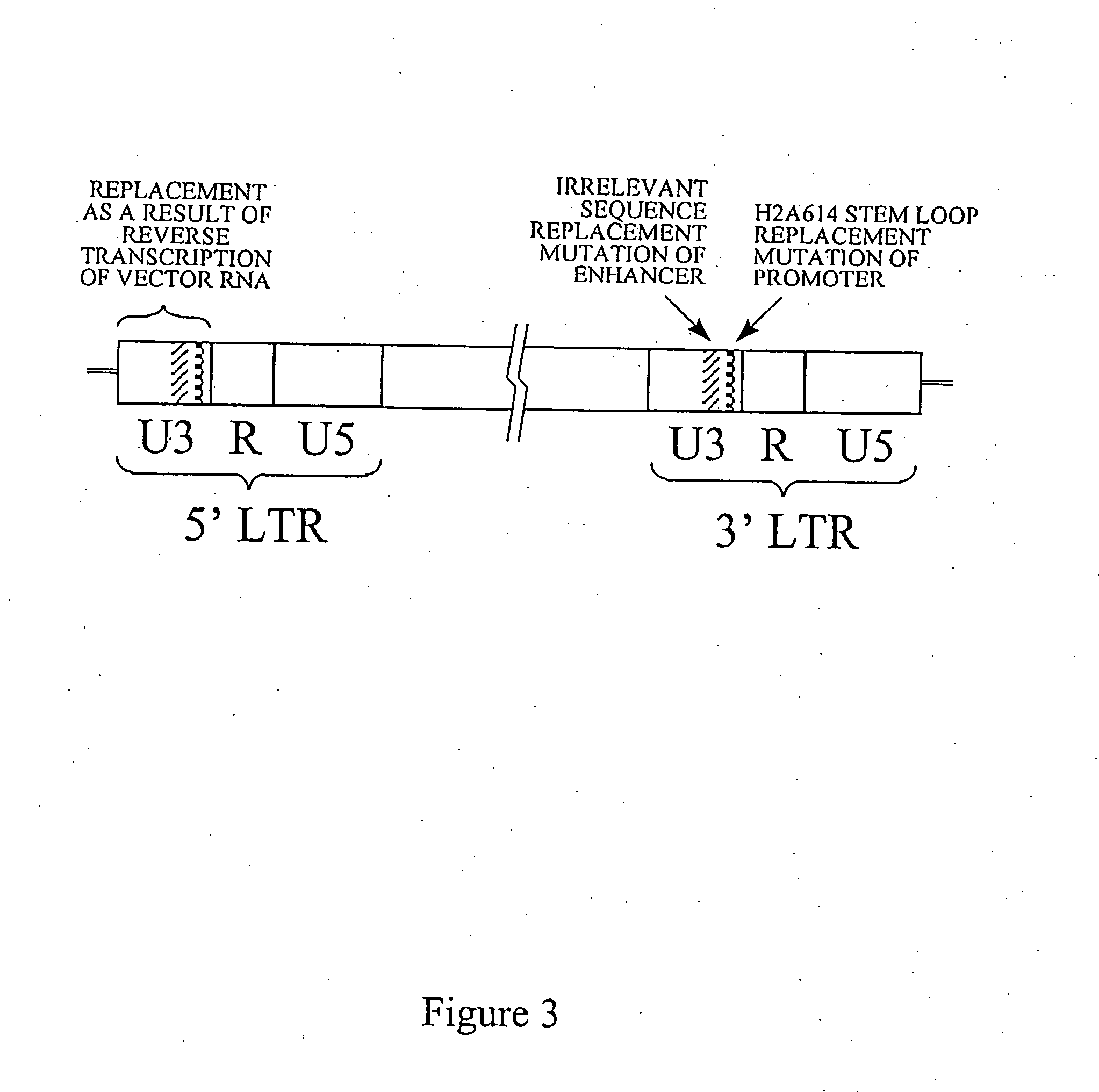
[0146] The nucleic acid sequence of Heterologous Vector retrovirus present in a plasmid that contains a Neo gene in a region outside of the retrovirus vector nucleic acid sequence can be modified by (FIG. 3) by replacement of a 188 base pair region of the 3′ enhancer with 188 base pairs derived from the bacterial Neo gene as described in Example 1. By the same methods, the promoter sequence can be replaced with sequences for a stem loop processing signal derived from mouse histone H2A614 gene. Retrovirus vectors containing these modifications can be produced by transfection of packaging cells with this plasmid vector and selection of a producer cell line. Such retrovirus vectors can be used for delivery of an Exogenous Nucleic Acid to a target cell wherein mRNA expressed from Exogenous Nucleic Acid can be polyadenylated by using the downstream el...
example 3
A Retroviral Vector with Inactivated Promoter / Enhancer Which Contains a Non-Native Polyadenylation Signal (the Human G-CSF Gene with the AATAAA and mRNA Destabilization Elements Removed).
[0147] A Heterologous Vector (retrovirus vector) can be constructed in which the 3′ LTR promoter and enhancer were inactivated wherein the endogenous retroviral polyadenylation site is used. Modifications to provide inactivation are made to a retroviral vector nucleic acid sequence present in a plasmid (pENZ-1). The region of the LTR containing the promoter / enhancer and the endogenous retroviral polyadenylation signal upstream from the AATAAA element was replaced with a portion of an efficient exogenous polyadenylation signal. In this way, vector mRNA can be polyadenylated by using the retroviral downstream AATAAA element. Here, a polyadenylation processing signal from the human G-CSF gene with the AATAAA and mRNA destabilization elements removed can be used to replace a region of the 3′ U3 that en...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| adsorption | aaaaa | aaaaa |
| nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com