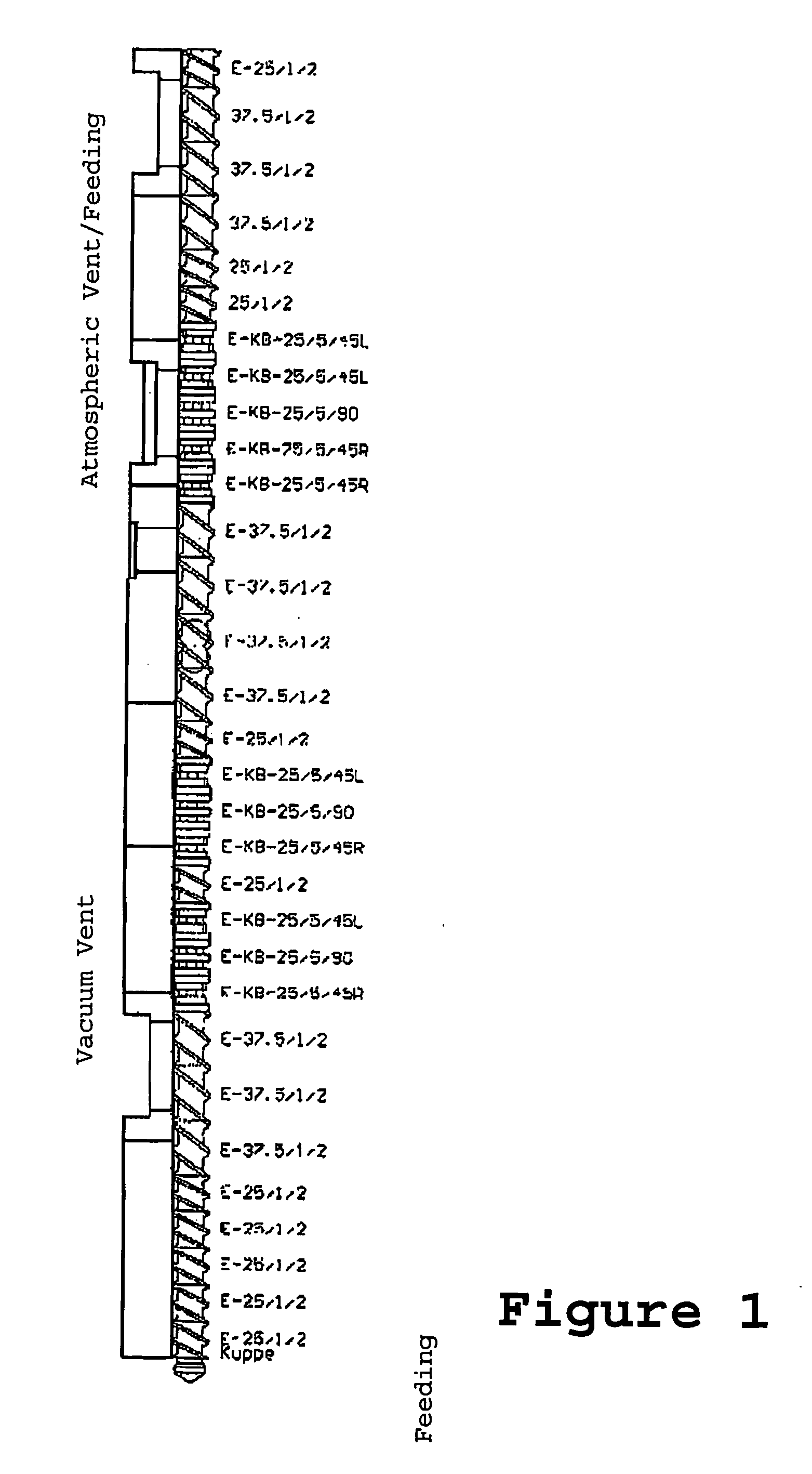
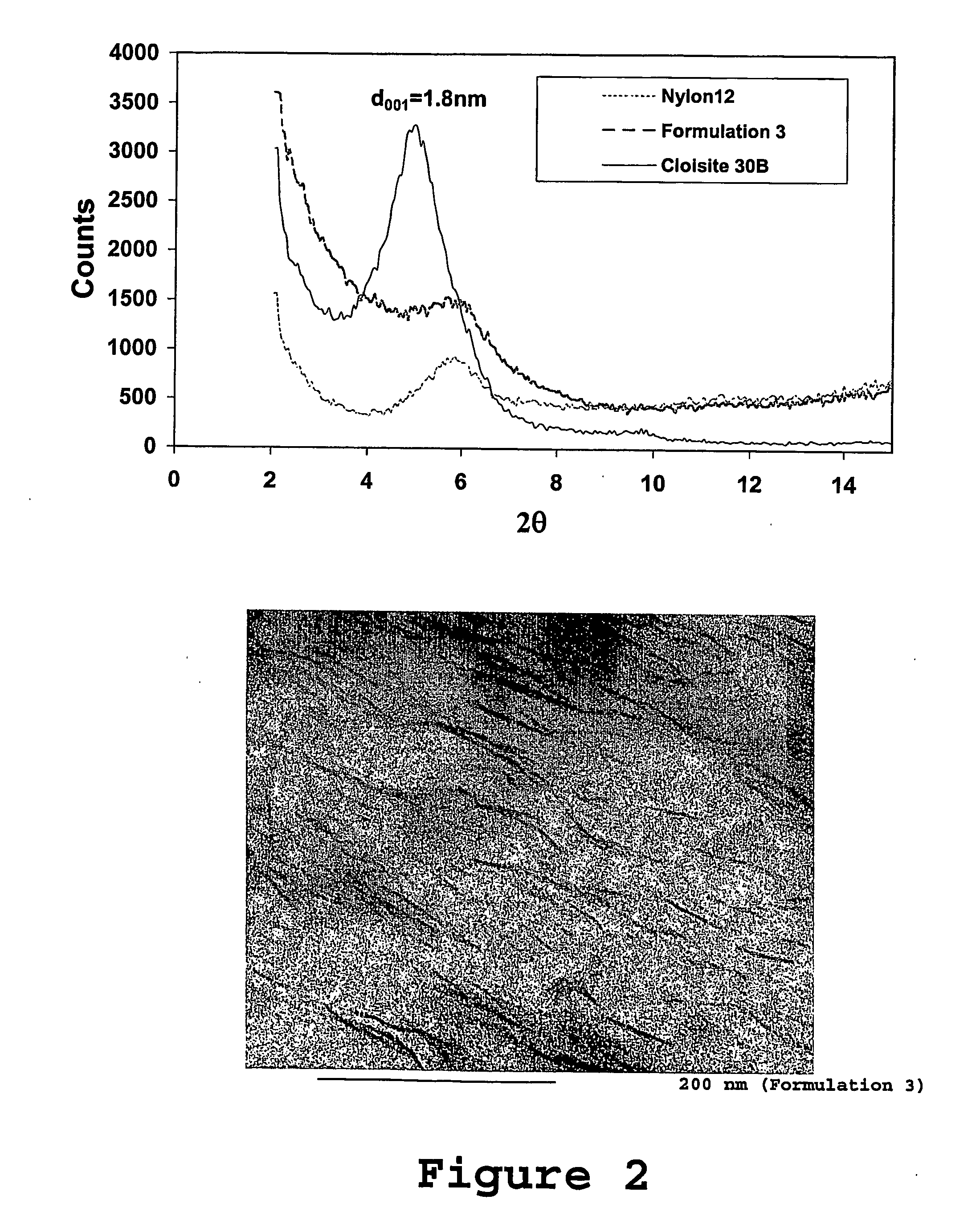
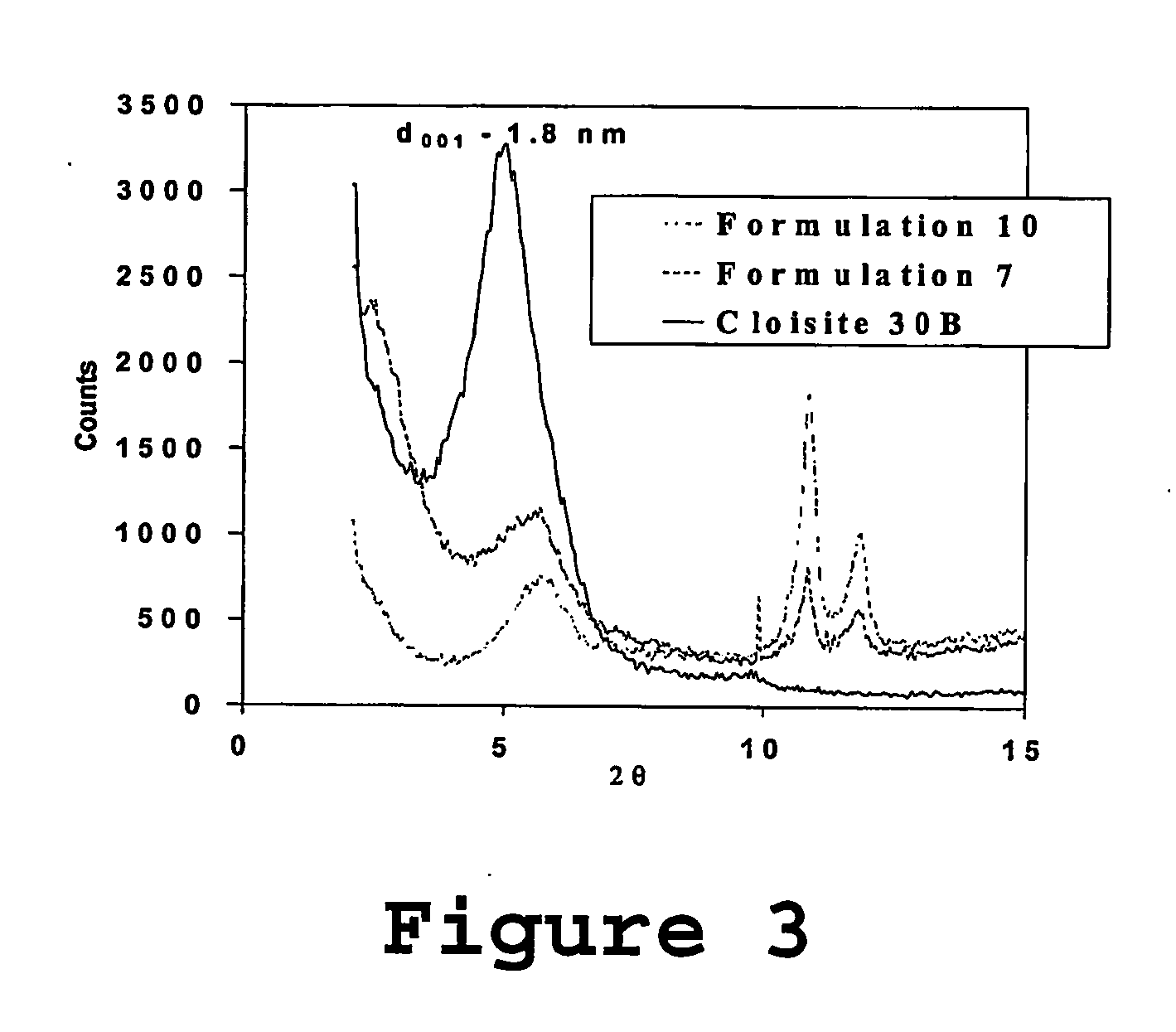
Fire resistant material
a technology of fire-retardant materials and materials, applied in the field of fire-retardant materials, can solve the problems of limiting their suitability, putting pressure on the use of halogenated compounds and certain, and considerable recent impetus to reduce the use of flame-retardant classes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Melamine Hydrochloride Modified Montmorillonite (IOH1)
[0115] Montmorillonite exchanged Na+ (Cation Exchange Capacity (CEC)=92 meq / 100 g) was suspended in 80° C. DI water (2% w / w) and mechanically stirred at 1500 rpm for 60 min. Melamine monohydrochloride salt (1.4 mmol / 100 g montmorillonite) was then added to the solution and the resultant suspension allowed to cool with continued stirring for a further 150 min. Following filtration of the suspension, the precipitate was thoroughly washed with warm DI water and then preliminary dried (60-80° C.). The resultant granular organically modified clay was ground to a particle size of less than 50 micron and then further dried at 75° C. prior to processing or analysis.
XRD (CuKα1 source λ = 0.154 nm)Melamine•HCl modifiedCationNa+MontmorilloniteXRD d0011.10 nm1.27 nm
[0116] Results indicate that with ion exchange montmorillonite's intergallery spacing is increased from 1.10 nm to 1.27 nm. This result is consistent with sodium...
example 2a
Preparation of Melamine Hydrochloride Modified Montmorillonite in the Presence of Melamine (IOH2)
[0117] Montmorillonite exchanged Na+ (Cation Exchange Capacity (CEC)=92 meq / 100 g) was suspended in 80° C. DI water (2% w / w), melamine added (1.4 mmol / 100 g montmorillonite) and the solution mechanically stirred at 1500 rpm for 60 min. Melamine monohydrochloride salt (1.4 mmol / 100 g montmorillonite) was then added to the solution and the resultant suspension allowed to cool with continued stirring for a further 150 min. Following filtration of the suspension, the precipitate was thoroughly washed with warm DI water and then preliminary dried (60-80° C.). The resultant granular organically modified clay was ground to a particle size of less than 50 micron and then further dried at 75° C. prior to processing or analysis.
XRD (CuKα1 source λ = 0.154 nm)Melamine and Melamine•HCl modifiedCationNa+montmorilloniteXRD d0011.10 nm1.39 nm
[0118] Results indicate that montmorillonite modified by m...
example 2b
Preparation of Melamine Hydrochloride Modified Montmorillonite in the Presence of Melamine (IOH2)
[0119] 3.0 Kg of sodium montmorillonite was dispersed into 200L de-ionized water at 60° C. with vigorous stirring (200 rpm) adding the powder slowly over a period of approximately one hour to assist wetting out of the individual particles / platelets. After the suspension had stirred at that temperature for approximately 2 hours, an aqueous solution (35L) containing 1.39 Kg melamine and 0.92L HCl (9.65M) at 85° C. was rapidly added whilst the impeller speed was simultaneously increased to 300 rpm. After an initial period of high viscosity whilst the modified montmorillonite aggregated, the viscosity decreased and the clay solution was allowed to stir for a further 3 hours at 60° C. Following filtration of the suspension the collected modified clay was re-dispersed into de-ionized water (150L) and allowed to stir for 1 hour at 60° C. before an aqueous solution (10 L) containing 0.385 Kg me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com