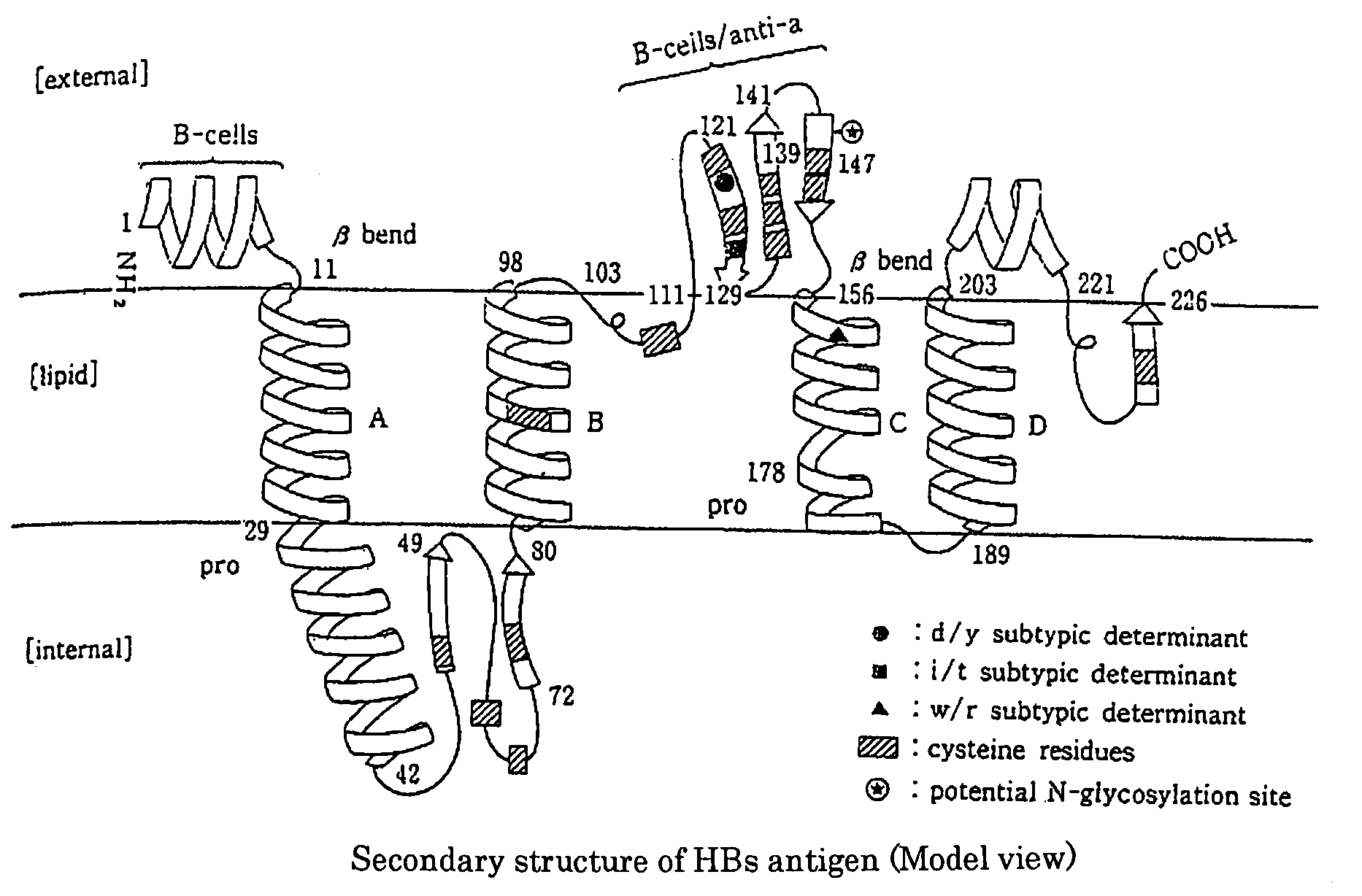
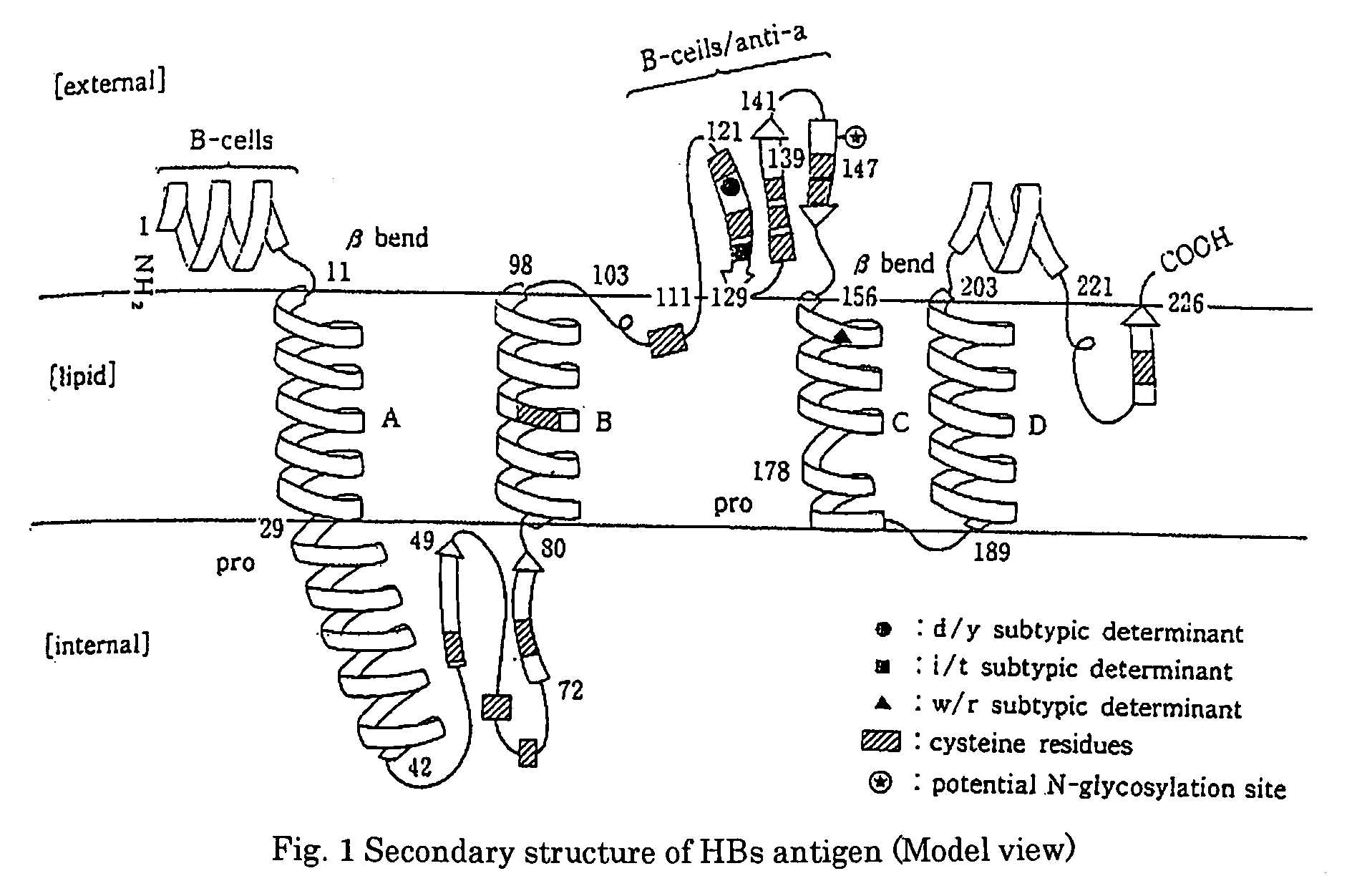
Method of Detecting Hepatitis B Virus S Antigen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression and Purification of TrpE-HBs (26 to 80) Antigen
(A) Construction of TrpE-HBs (26 to 80) Antigen-Expressing Plasmid
[0053]An expression plasmid for HBs (26 to 80) region was constructed by the following method. 100 μl serum from an HBV patient was mixed with 100 μl DNA extract [10 μl of 1 M Tris-HCl (pH 8.4), 8 μl of 250 mM EDTA, 40 μl of 10% SDS, 8 μl of 5 M NaCl, 10 μl of 20 mg / ml. Proteinase K, 1 μl tRNA (5 μg / μl), and 23 μl sterilized water] and incubated at 54° C. for 30 minutes. The sample was mixed with 200 μl phenol / chloroform (1 / 1) solution and then centrifuged at 15 Krpm for 5 minutes to give a supernatant, and 150 μl isopropanol and 7 μl of 5 M NaCl were added to the supernatant and left at −20° C. for 1 hour. After centrifugation at 15 Krpm at 4° C. for 5 minutes, the precipitates were rinsed with 70% ethanol and then centrifuged again at 15 Krpm at 4° C. for 5 minutes. The precipitates were air-dried and dissolved in 20 μl sterilized water to give an HBV DNA sol...
example 2
Preparation of Hybridoma
[0061]The polypeptide [trpE-HBs (26 to 80)] prepared by the method described above was dissolved with 6M urea and then diluted at a final concentration of 0.2 to 1.0 mg / ml in 10 mM phosphate buffer (pH 7.3) containing 0.15 M NaCl (PBS), then mixed with an equal volume of Freund's adjuvant, and administered intraperitoneally in a dose of 10 to 20 μg to a 4- to 6-week-old BALB / c mouse.
[0062]Booster was carried out every 2 to 4 weeks in the same manner as above, and for final immunization, 10 μg HBs dissolved in PBS was administered to the caudal vein.
[0063]At three days after the final immunization, the spleen was aseptically removed from the mouse, then broken into individual cells with scissors and a metallic mesh and washed 3 times with RPMI-1640 medium. Mouse myeloma cell strain Sp2 / OAg14 at the logarithmic growth phase was washed 3 times with RPMI-1640 medium, and the cells were mixed with the spleen cells at a ratio of 1:5. After centrifugation at 200×g f...
example 3
Preparation and Analysis of Monoclonal Antibody
[0066]Each of the hybridomas obtained by the method described in Example 2 was transplanted in a BALB / c mouse abdominal cavity previously administered with pristane, and the monoclonal antibody produced in the ascites was obtained.
[0067]The IgG fraction containing the monoclonal antibody was purified by affinity chromatography on a protein A Sepharose column.
[0068]The respective obtained monoclonal antibodies were analyzed for their target epitope by using the TrpE-HBs (26 to 80) antigen and synthetic peptides each consisting of 20 amino acids synthesized on the basis of a sequence derived from the HBs region, and as a result, it was found that as shown in Table 1, these monoclonal antibodies recognize an epitope (amino acid numbers: 26 to 80) of the HBs antigen, which is located in the inside of a lipid bilayer.
[Table 1]
[0069]
TABLE 1(Poly)peptideAmino acidMonoclonal antibody namenamenumber4A36G61C10HBS-1 1-20−−−HBS-211-30−−−HBS-321-40−...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com