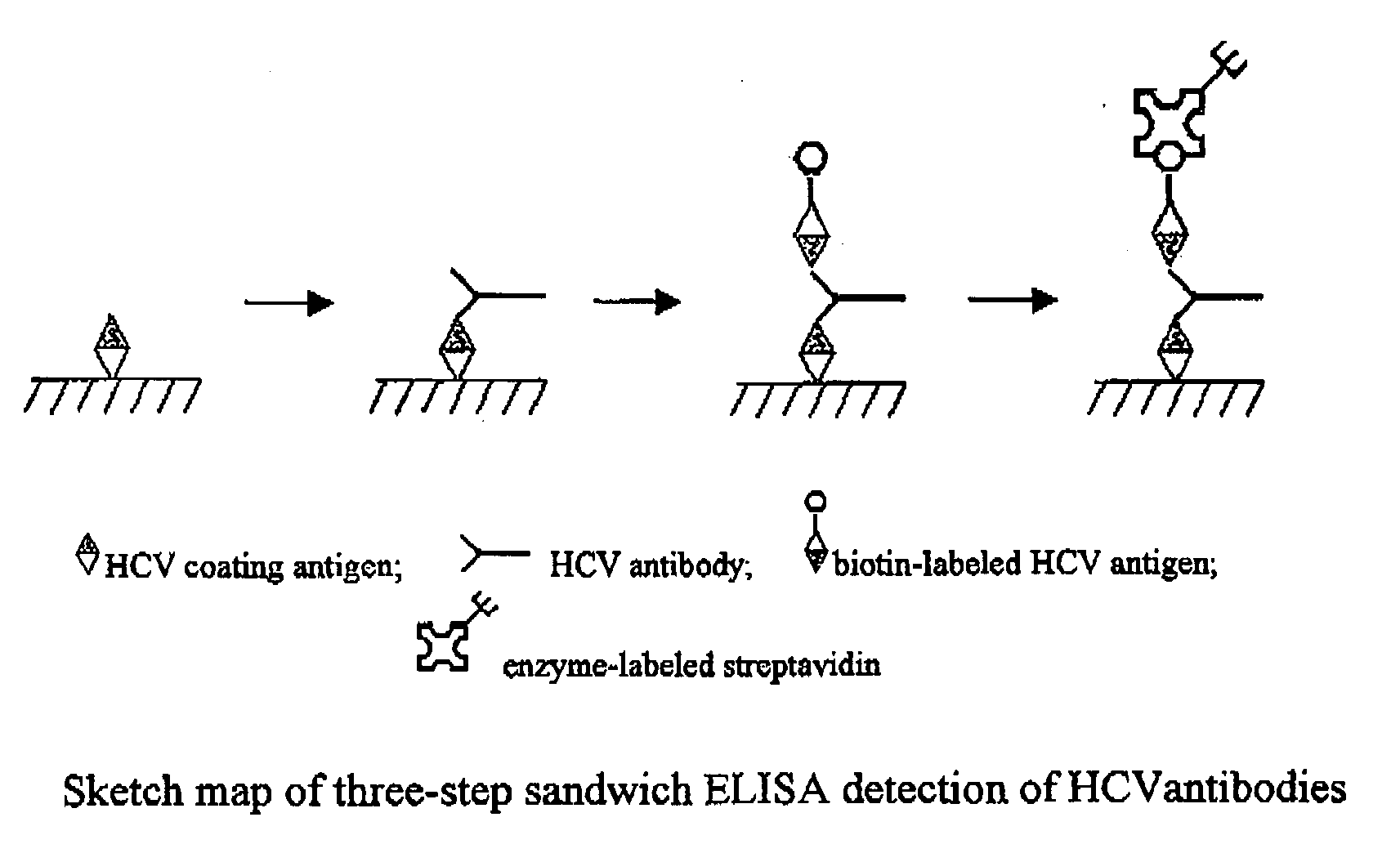
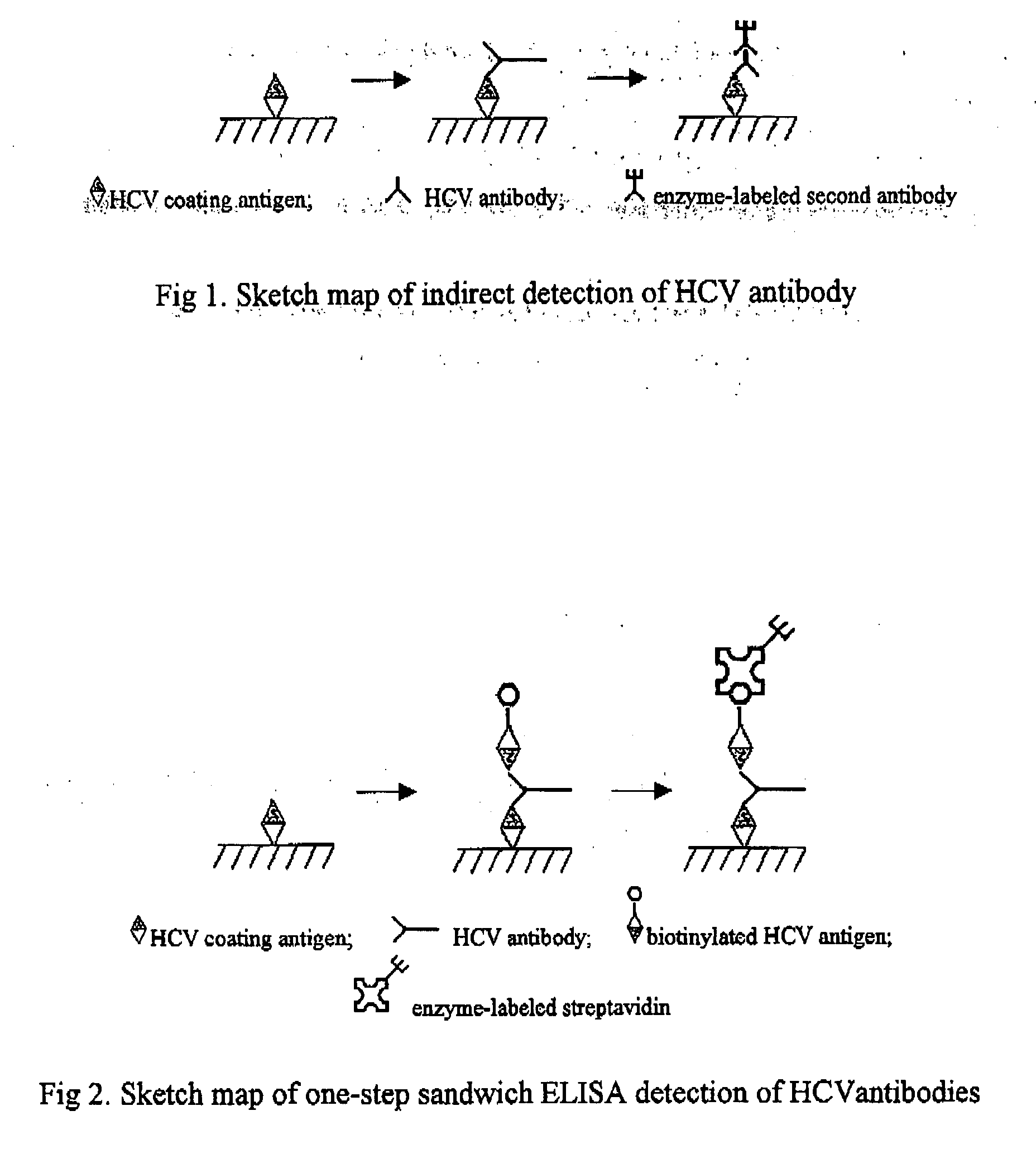
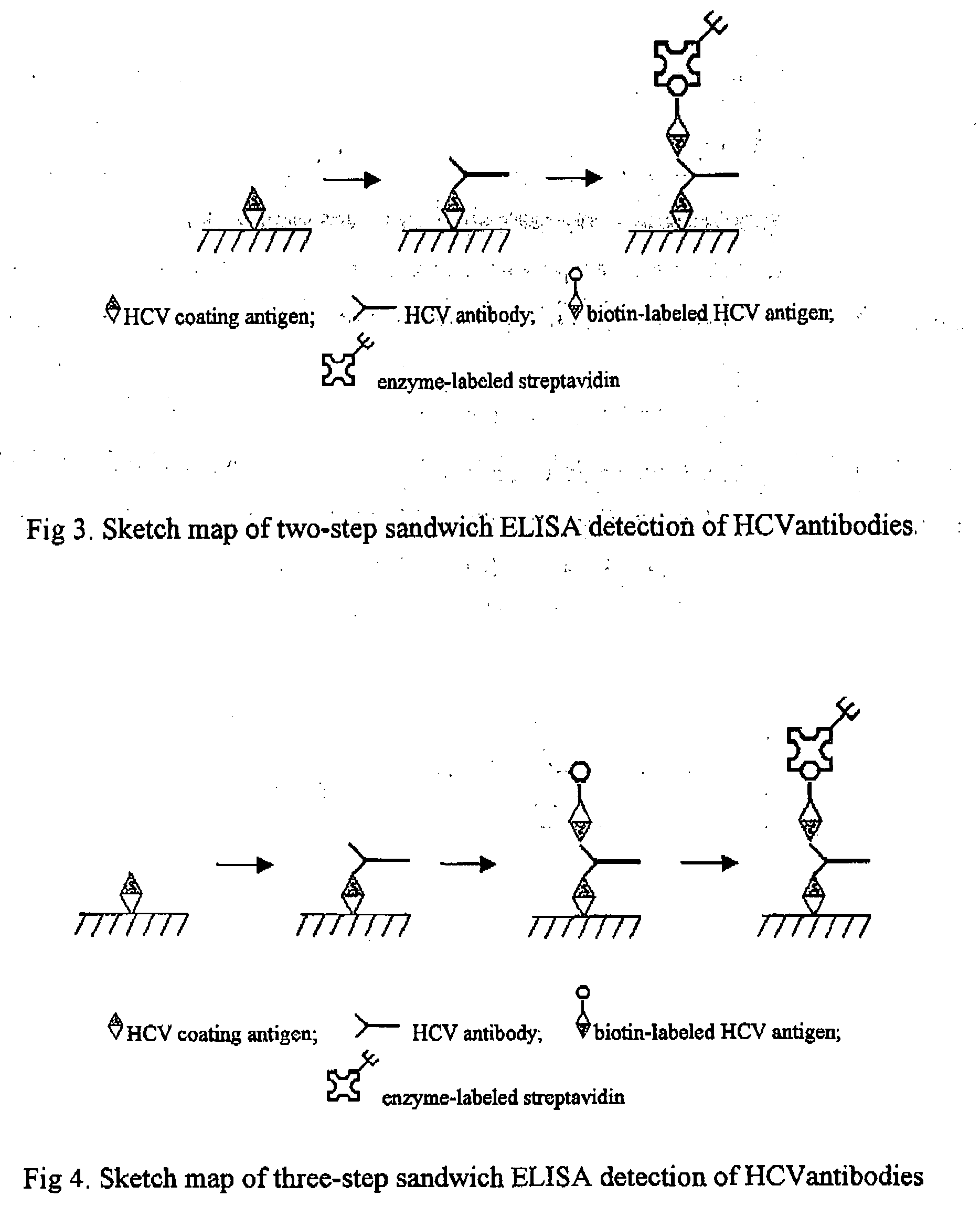Kit and Method for the Detection of Anti-Hepatitis C Virus (Hcv) Antibodies
a technology of anti-hepatitis c virus and kit, which is applied in the field of virus diagnostics, can solve the problems of affecting human health, bringing heavy burden to the bur society, and not satisfying sensitivity and specificity, so as to reduce the activity of hcv antigens, deactivate enzymes, and influence the structure of enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of Secondary Antigen and Enzyme Label
1. The Preparation of Biotinylation Secondary Antigen
[0082]We amplify the gene encoding HCV antigen a.a.1-28 (the 1st to 84th nucleotide in SEQ ID NO.1), the forward primer thereof has BamHI site, and the reserve one has BgIII and EcoRI sites between which there is a termination codon TAA. Then we amplify segment L (SEQ ID NO.3) encoding biotin acceptor protein by PCR, sense primer thereof has BamHI site, and anti-sense has BgIII site and EcoRI site between which there is a termination codon TAA.
[0083]Gene coding a.a.1-28 cleaved by restriction endonuclease BamHI and EcoRI is cloned into P2-G expression vector cleaved by BgIII and EcoRI endonuclease, the resulting positive clone P2-G-CORE (a.a.1-28) is named P2-HDVAg2, and the antigen expressed is named as HCVAg2. Clone gene L cleaved by BgIII / EcoRI into P2-G-CORE (a.a.1-28) treated by same endonucleases, we can obtain the positive clone P2-G-CORE (a.a.1-28)-L, named P2-X.
[0084]We ext...
example 3
SELECTION OF THE CONCENTRATION OF REDUCING AGENT
[0091]First, respectively use HCVAg1 and HCVAg as coating antigen, add different final concentration of β-mercaptoethanol in coating buffer, and detect using indirect method, (data in tab 1). We get similar result by adding different final concentration of β-mercaptoethanol in blocking buffer. The results show that reducing agent can increase HCV antigenic activity to a certain extent, indicating that there have some mismatching disulfide bond in the HCV antigen. The reducing agent can break up the mismatching disulfide bond of HCV antigen, and restore the natural conformation of the HCV antigen. The β-mercaptoethanol optimization concentration range is 0.5-5‰, prefers 1‰.
TABLE 1The effect of β-mercaptoethanol in coatingbuffer to sensitivity of HCV indirect kitfinal concentration of20‰10‰5‰2‰1‰0.5‰0.2‰0.1‰0β-mercaptoethanol incoating bufferaverage OD mean5.105.625.896.316.546.195.785.125.20value / Co of thepositive serumsIndication: Cut ...
example 4
PRODUCTION AND TEST OF THE PRESENT INVENTION KIT
1. Using Biotin Labeled HCV Antigen as the Second Antigen in the Kit
[0099]Dilute the HCV coating antigen with bicarbonate buffer (50 mM, pH9.51, contains 1‰β-mercaptoethanol (Sangon, Article No. M0482)) by a certain ratio, add 100 ul to each well of Polystyrene plate (Shenzhen jincanhua industry Co., Ltd irradiation Polystyrene microliter), Tween-20, pH7.4) washing buffer to wash it twice, then we desiccate the wells by patting. Add 120 ul pH7.4, 10 mM PB blocking buffer into each well, which contains 30% fetal calf serum (Beijing Yuanhengshenma Biotechnology Research Institute), 8% sucrose, 5‰casein (America Sigma-Aldrich Inc., article no. C-8645), 1‰β-mercaptoethanol (Sangon, Article No. M0482), 150 mM NaCl, and then block for 2 hours at 37° C. We then discard liquid in wells and desiccate the wells by patting. After dried by air in room equipped with draft equipment where temperature is 20-25° C. and humidity is 55%-65%, the plate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com