Electrode for electrolysis and electrolysis unit
a technology of electrolysis and electrolysis unit, which is applied in the direction of electrolysis components, electrolysis coatings, multiple component coatings, etc., can solve the problems of high cost of the device itself, ozone water having a desired concentration is difficult to efficiently form, and the device is liable to become complicated, so as to achieve the effect of reducing production cost, increasing ozone forming efficiency, and increasing energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
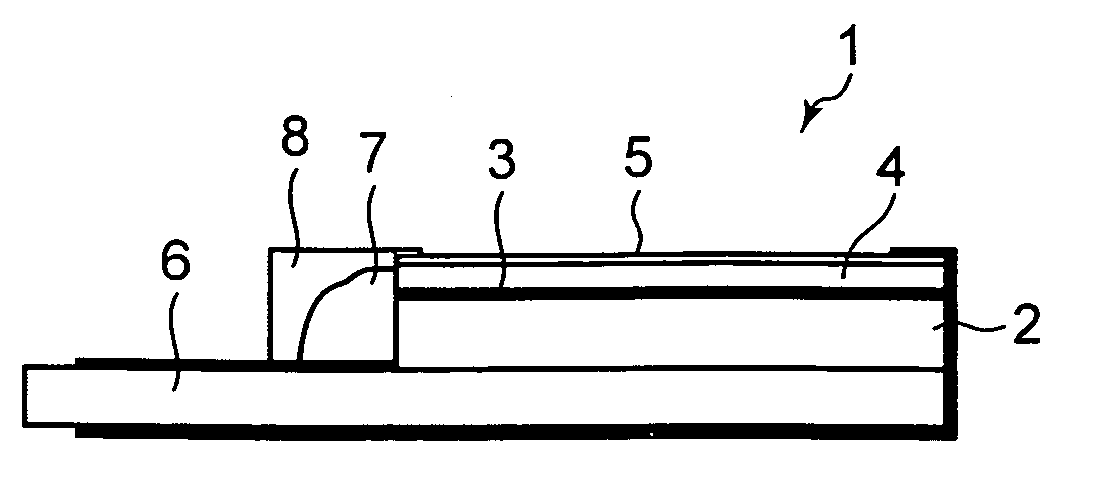
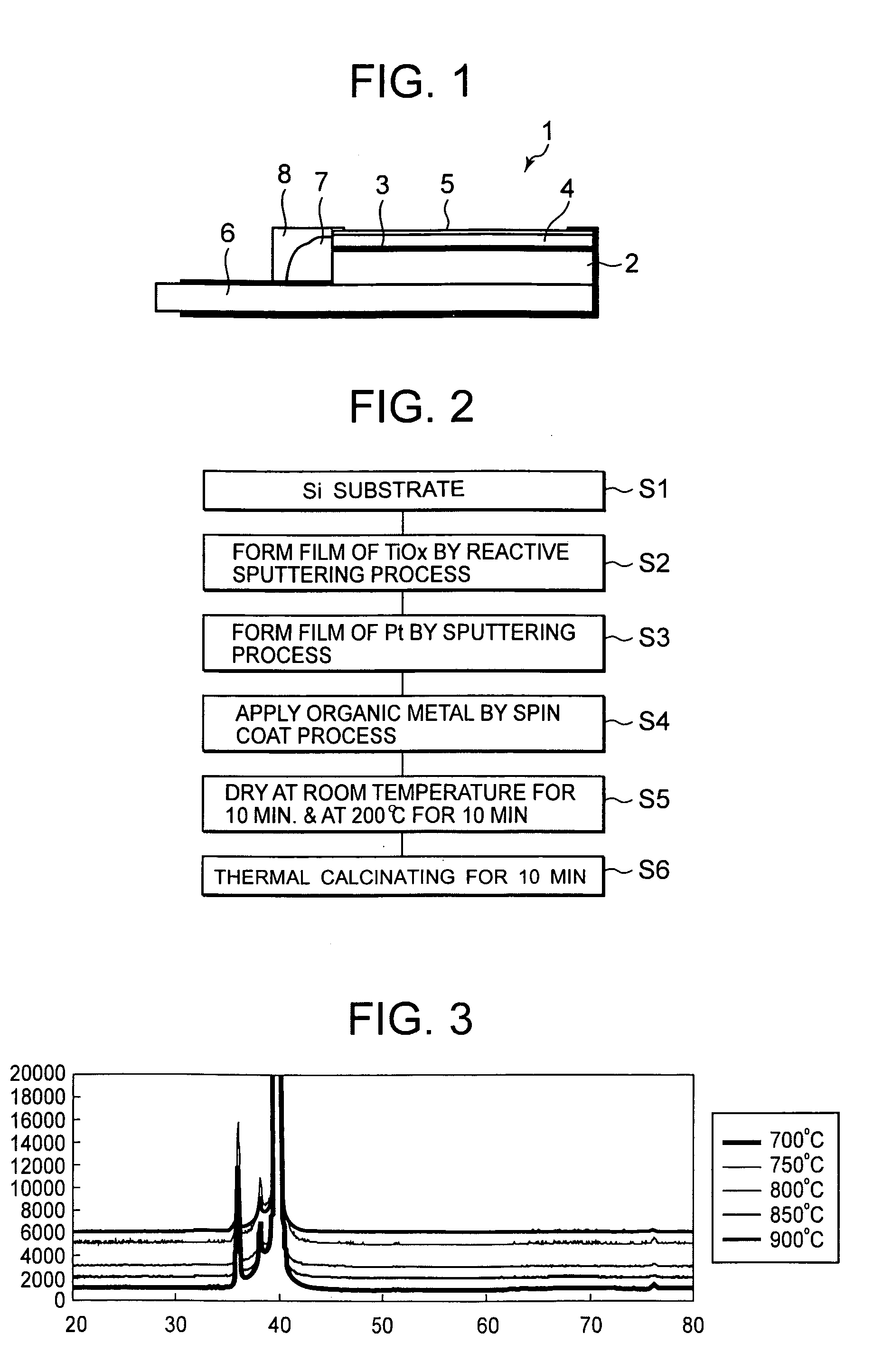
[0048]Next, a manufacturing method of an electrode 1 for electrolysis according to Example 1 of the present invention will be described with reference to a flow chart of FIG. 2. First, silicon (Si) constituting a substrate 2 is pretreated in step S1. Here, it is preferable that phosphorous (P), boron (B) and the like are introduced as impurities into Si to improve the conductivity. Si having a very flat surface is used. It is to be noted that in the present example, Si is used as the substrate 2, but a conductive material may be used.
[0049]In the pretreatment, the substrate 2 of Si is treated with 5% of hydrofluoric acid to remove a native oxide film formed on the surface of the substrate 2. In consequence, the surface of the substrate 2 is further flattened. It is to be noted that the pretreatment does not have to be performed. Afterward, the surface of the substrate 2 is rinsed with pure water, and then in step S2, the substrate is introduced into a chamber of an existing sputteri...
example 2
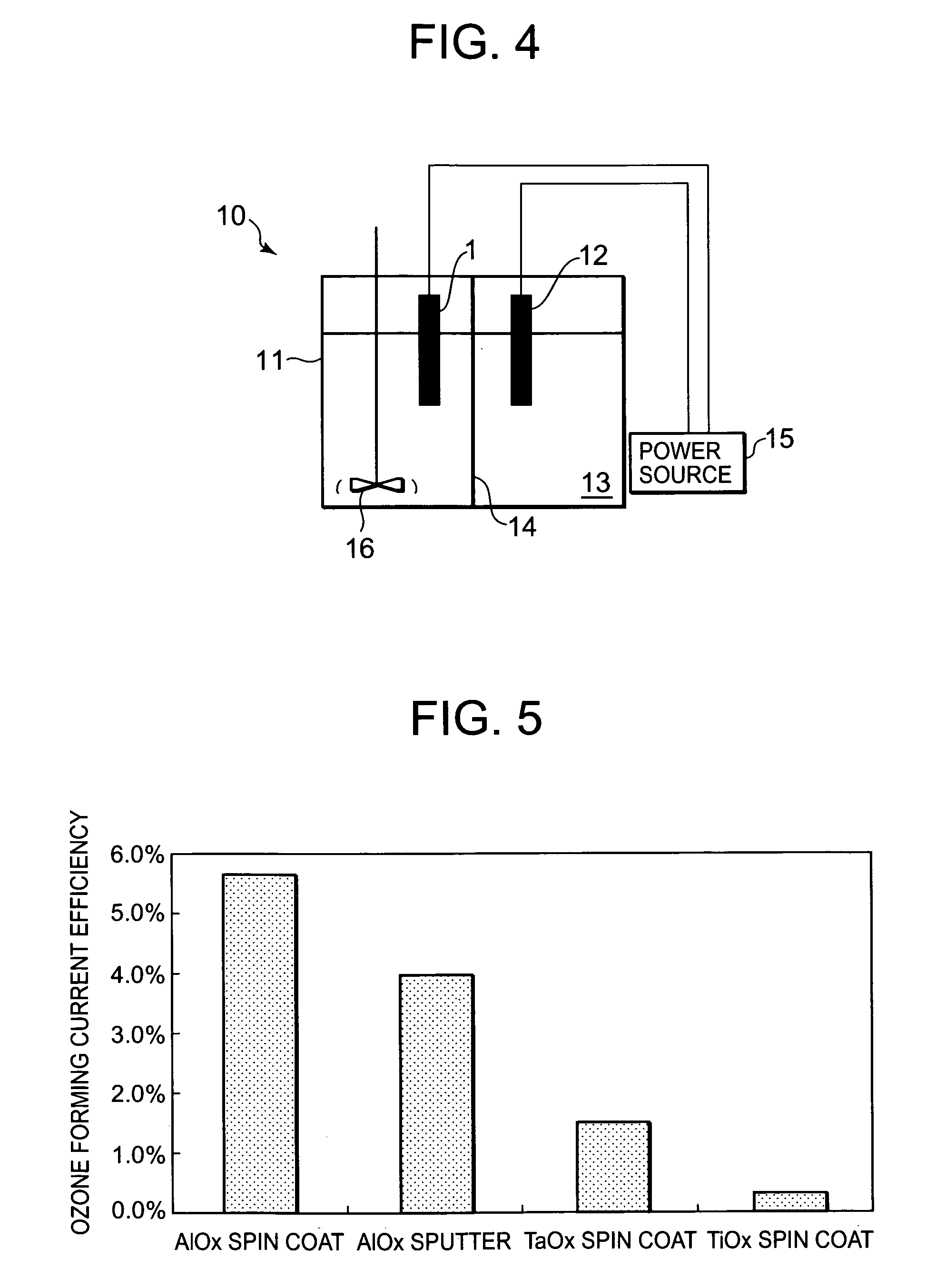
[0083]Next, a manufacturing method of an electrode 21 for electrolysis according to Example 2 of the present invention will be described with reference to a flow chart of FIG. 6. It is to be noted that FIG. 7 is a schematic constitution diagram of the electrode 21 for electrolysis obtained by the example. First, in step S11, Si constituting a substrate 22 is pretreated in the same manner as in the above example. A material of the substrate 22 is similar to that of the above example, so that description thereof is omitted. Subsequently, in step S12, the substrate is introduced into a chamber of an existing sputtering device to form a film.
[0084]In the step S12, a close contact layer 23 for improving a close contact property of an intermediate layer 24 is formed on the surface of the substrate 22 as described above. The close contact layer 23 is formed on the substrate 22 by a reactive sputtering process in the same manner as in the above example. The close contact layer 23 is made of...
example 3
[0101]Next, an electrode for electrolysis according to Example 3 of the present invention will be described. It is to be noted that a manufacturing method of an electrode 31 for electrolysis obtained according to such an example is similar to that shown in the flow chart of FIG. 2 in Example 1, and a schematic constitution diagram is substantially similar to FIG. 1, so that detailed description of the manufacturing method is omitted.
[0102]That is, in the electrode for electrolysis according to the example, a close contact layer 3 of titanium oxide is formed on the surface of Si constituting a substrate by a sputtering process as described above, and an intermediate layer 4 of platinum is formed on the surface of the close contact layer 3 by the sputtering process.
[0103]Subsequently, a surface layer 5 is formed on the surface of a substrate 2 provided with the intermediate layer 4. In such an example, the surface layer 5 is formed by a spin coat process, so that the surface of the su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com