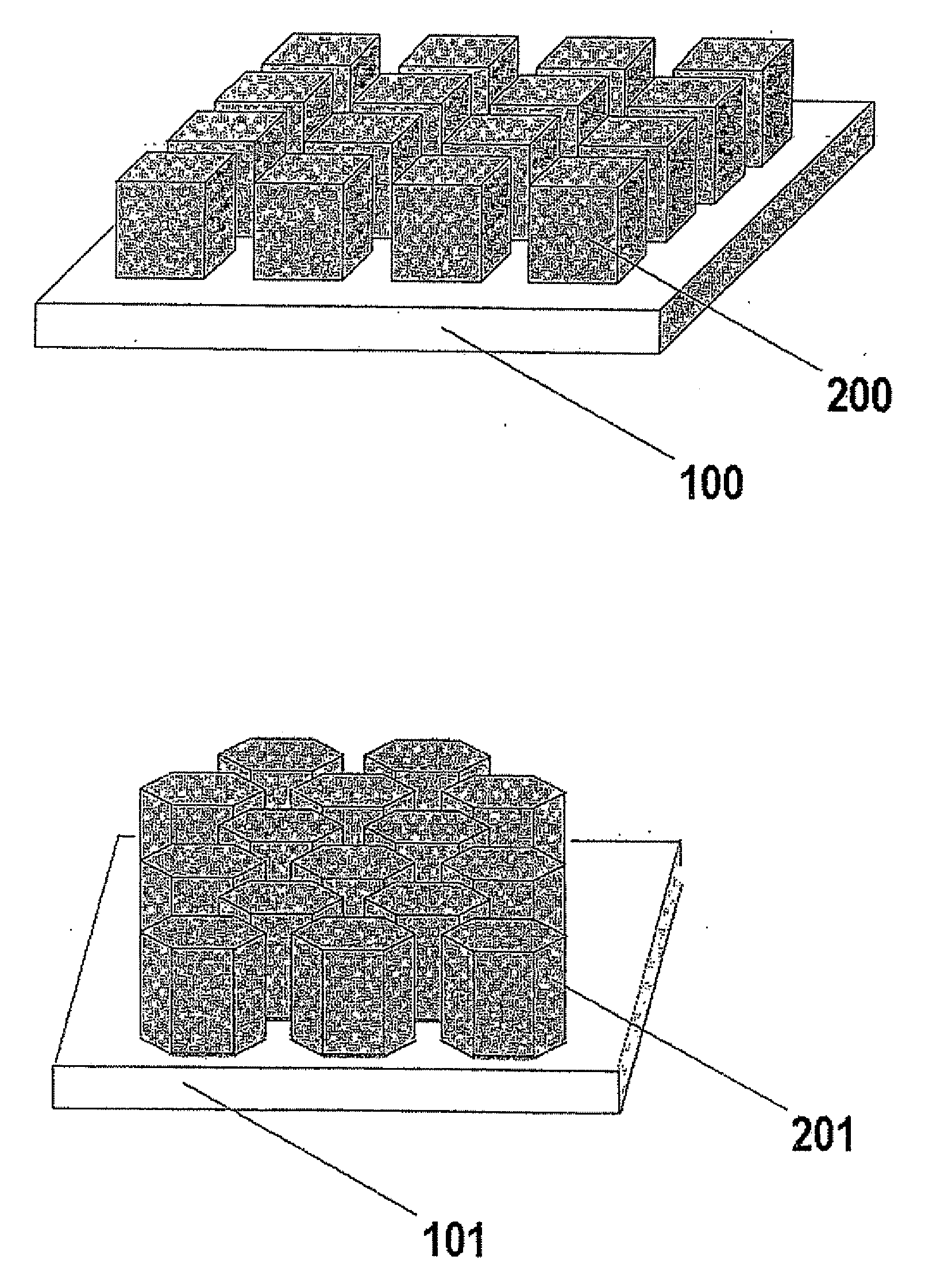
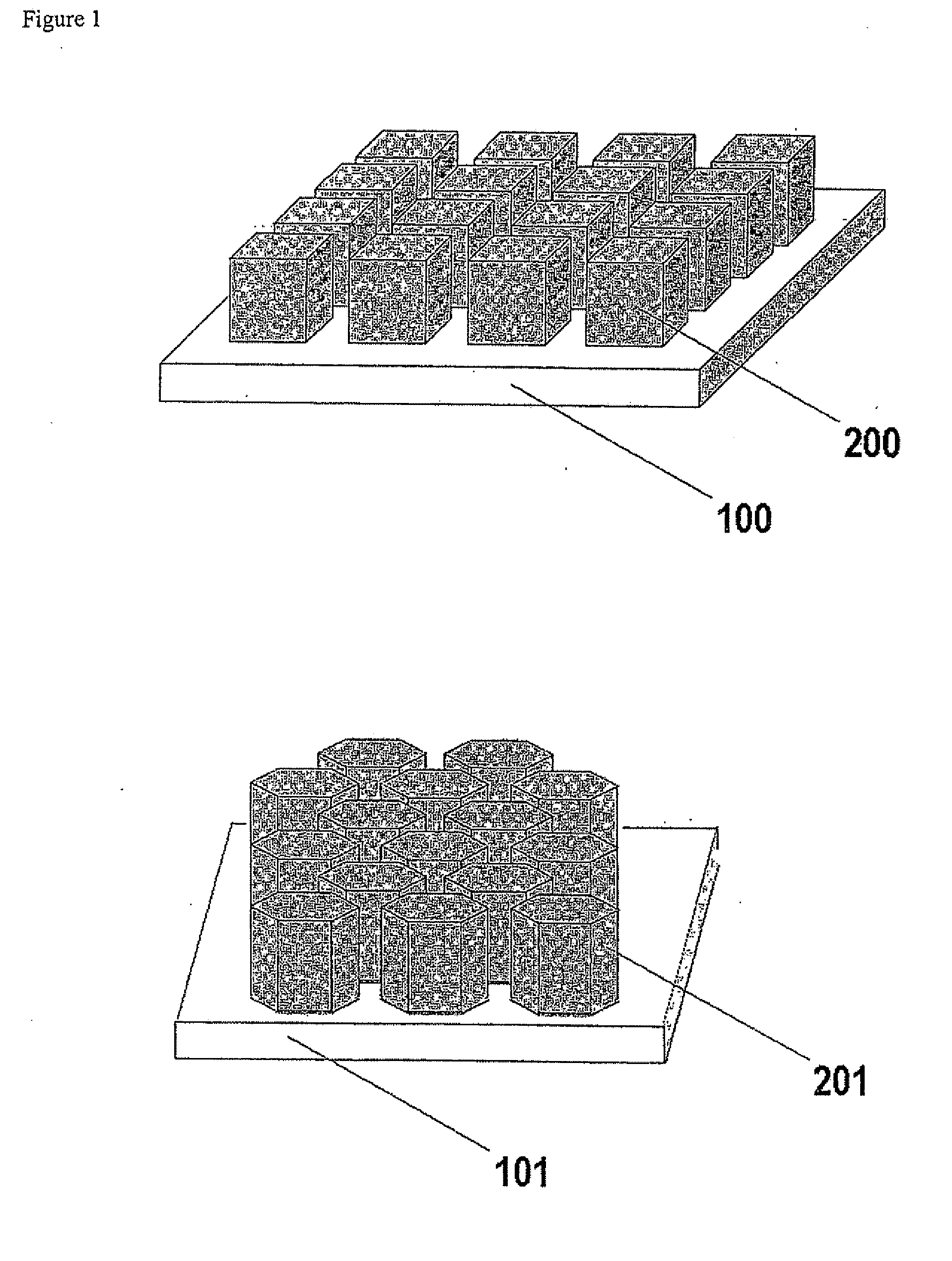
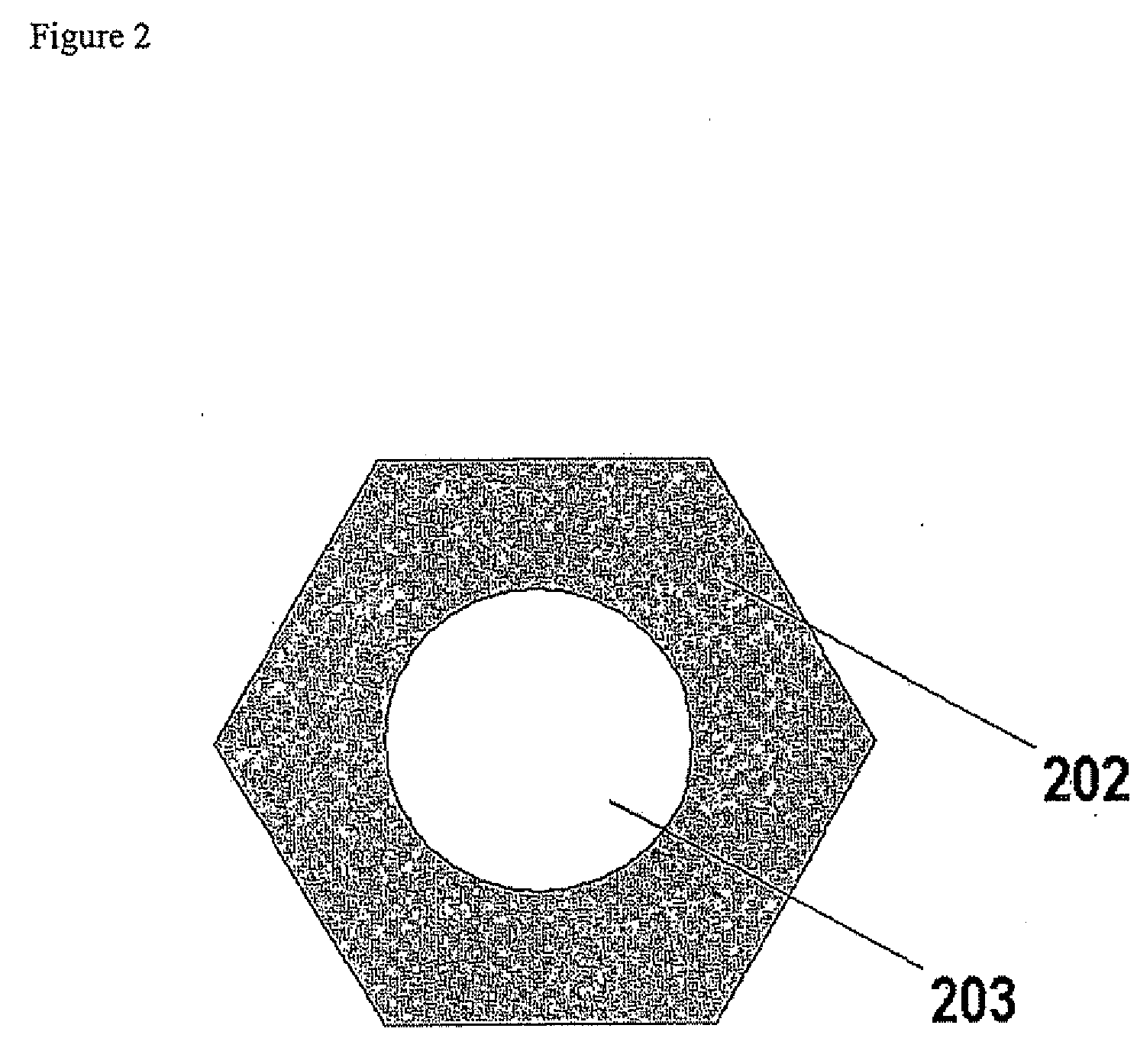
Gas Diffusion Electrodes, Membrane-Electrode Assemblies and Method for the Production Thereof
a technology of gas diffusion electrodes and membrane electrodes, which is applied in the manufacture of cables/conductor parts, electrical equipment, fuel cells, etc., can solve the problems of cumbersome scale up, difficult control of exchange membranes, and common physical and chemical vapor deposition techniques (pvd or cvd), and achieves a surprising enhancement of catalyst utilization factor, uniform local permeability of coatings, and increased useful catalytic surface effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032]A series of gas diffusion electrodes was prepared according to the Example of the cited U.S. Provisional Patent Application Ser. No. 60 / 580,739, the difference being that a series of masks were interposed between the gas diffusion medium and the IBAD source to obtain patterned noble metal coatings. Platinum was selected as the noble metal to apply, as in the case of the cited application. A gas diffusion medium was selected consisting of a three-dimensional woven carbon cloth coated with a mixture of Shawinigan Acetylene Black carbon particles and PTFE, for a total thickness of 410 microns, a basis weight of 210 g / m2, a density of 0.54 g / cm3, an electrical resistivity of 525 mΩcm, an air permeability of 0.84 Gurley, a porosity of 25 microns with a mean pore size of 6 microns and an average smoothness of 5000 Gurley seconds. The gas diffusion medium so obtained was divided into equivalent pieces, each of which were subjected to dual IBAD deposition of platinum metal after overl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com