High rate electrochemical devices
- Summary
- Abstract
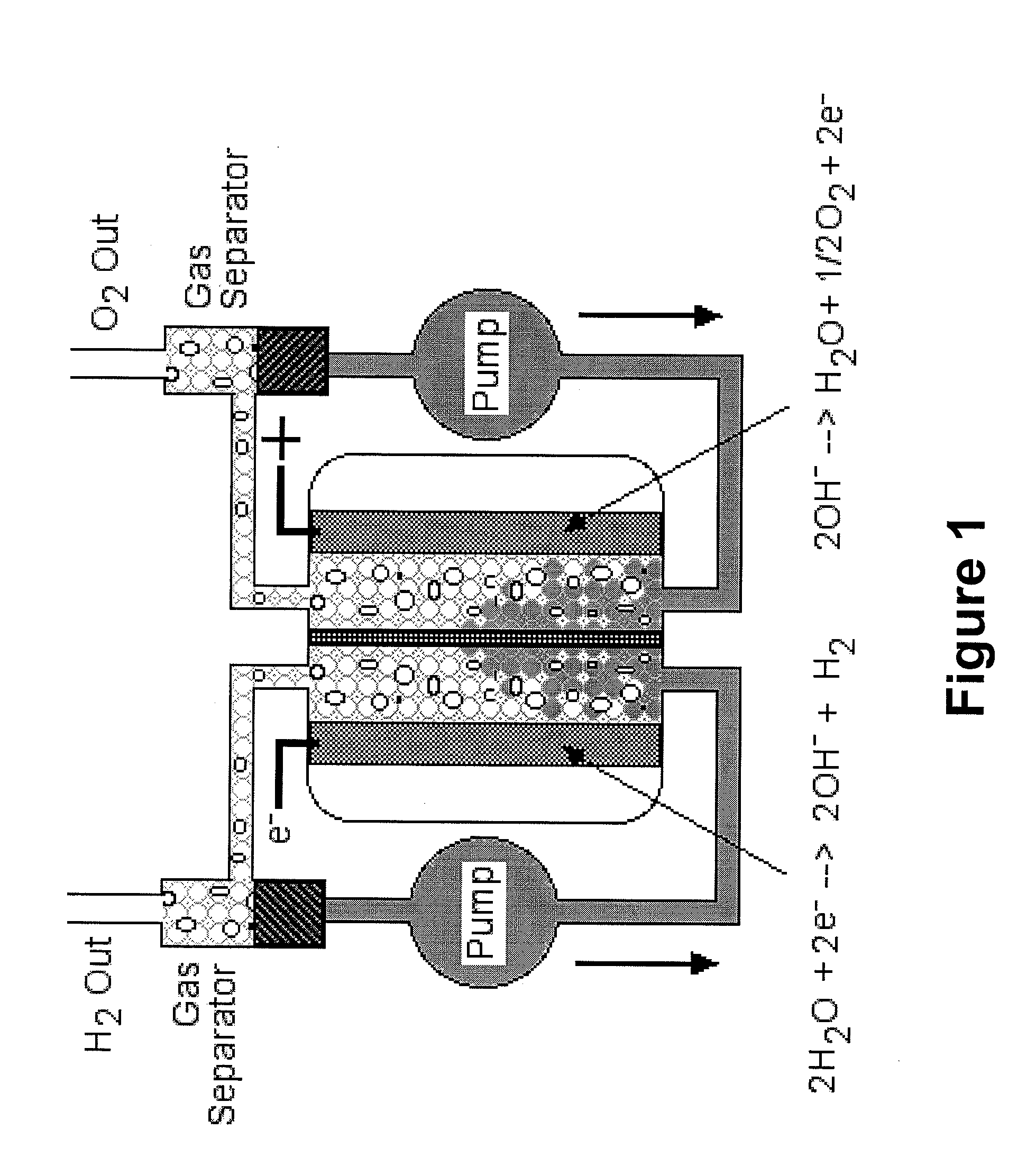
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of Nanoparticle Addition to Upper (Cathode) Chamber
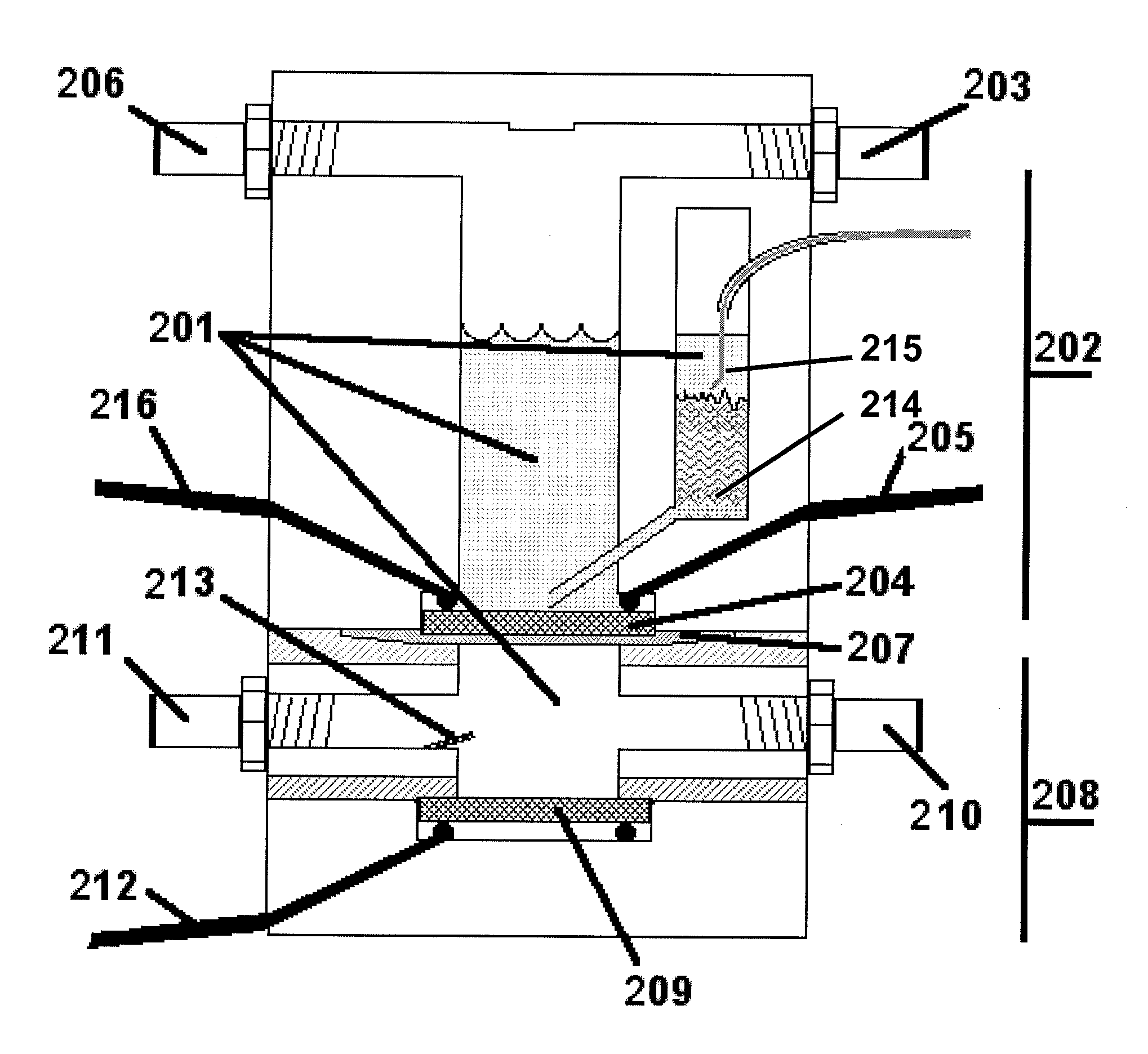
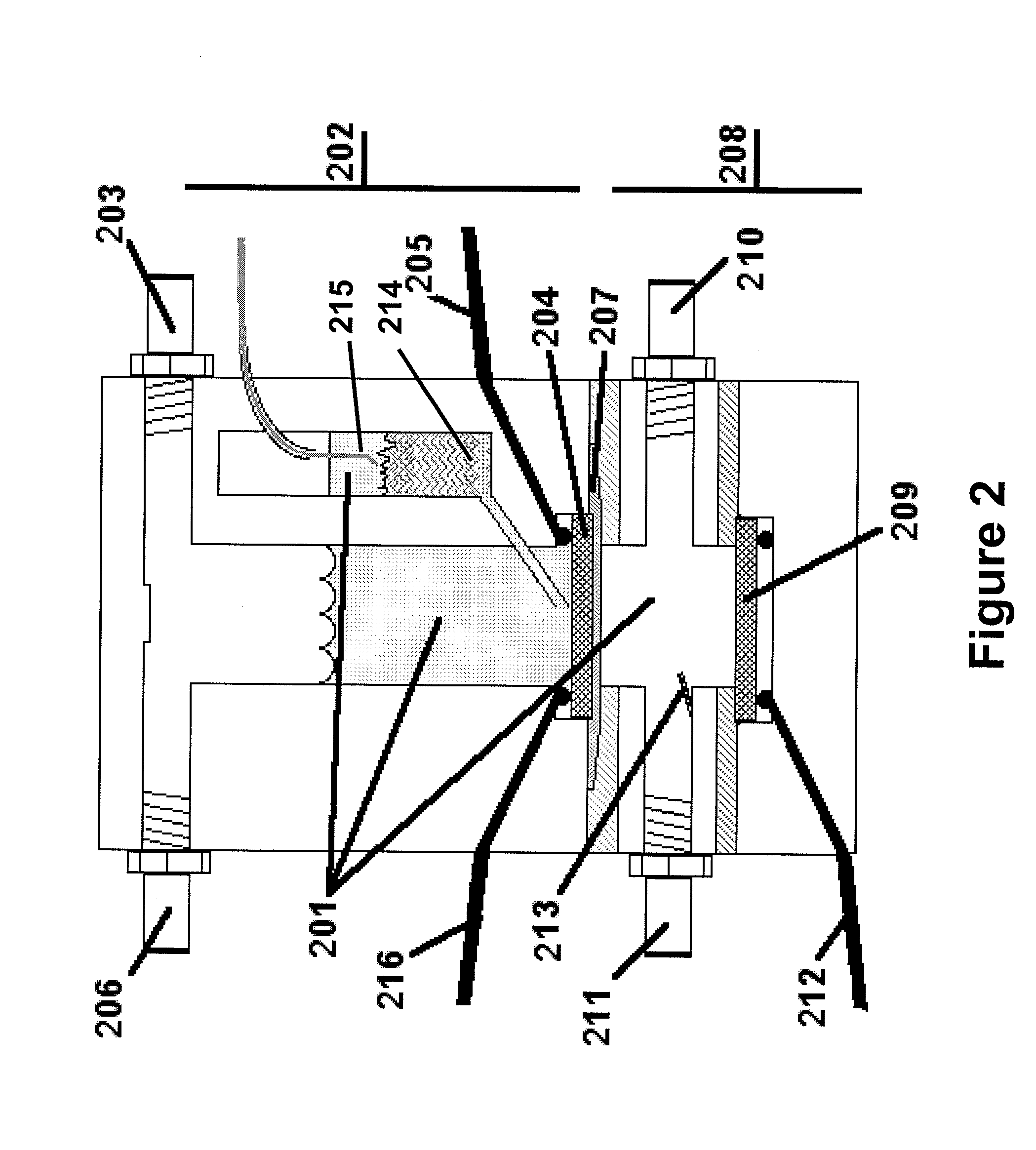
[0066]The water electrolysis device shown in FIG. 2 was used to perform the experiment. FIG. 7 illustrates the effect of injecting nano-catalyst into the upper chamber of the water electrolysis device. The electrolyzer is allowed to reach a steady-state voltage under 1 A / cm2, which is evident after about 60 seconds. At time 701, nickel nanoparticles (QSI-Nano® Nickel, 5-30 nm, from QuantumSphere Inc.) were added to the upper chamber of the electrolyzer. An efficiency increase of 10% was observed after addition of nickel nanoparticles 502. After about 15 minutes, steady state efficiency improvement was about 20%.
example 2
Comparison of Five Different Cathode Electrodes
[0067]FIG. 8 compares the performance of several different cathode electrodes for a water electrolysis device. Electrodes compared are Incofoam nickel metal foam (purchased from Inco), a sintered micron nickel plate (a sintered, compressed electrode of 1-5 micron Ni particles, purchased from Alfa Aesar), a sintered nickel plate of nickel particles (Inco 123, purchased from Inco), a sintered electrode of 5-30 nm nano-nickel from QuantumSphere Inc, and a sintered plate prepared from 1-5 micron nickel particles, as above, with 10 wt % 5-30 nm nickel from QuantumSphere Inc. injected into the electrolyte. An improvement over the base electrode 801 with no catalytic powders added was observed when micron particles were added 802 on a 1 Amp / cm2 load. This combination, however, agglomerated after less than an hour of running with significant degradation. The addition of nano-catalyst 803 increased the performance by nearly 4 times compared to t...
example 3
High Rate Capability of Electrolysis Device
[0068]FIG. 9 illustrates the improved rate capability of the electrolysis device described in the preferred embodiments relative to a more traditional system. This figure shows the voltage and current relationship of several electrode designs. Sets 901-904 are of a design compressed powders, as shown in Example 2. Sets 905-906 show the preferred embodiment. Specifically, data sets 901 / 901′ show a carbon electrode, 902 / 902′ show a smooth nickel electrode, 903 / 903′ show a compressed micron sized nickel electrode, 904 / 904′ show a micron nickel with nano sized powders added then compressed, 905 / 905′ show the preferred embodiment with no added catalyst, and 906 / 906′ show the most preferred embodiment (a foam nickel electrode with nano-nickel particles injected.) with nano-catalyst added. A voltage difference of 2 volts is about 75% efficiency. The last set of dots, 907, is a cell potential of 1,584 volts and represents over 90% efficiency when c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com