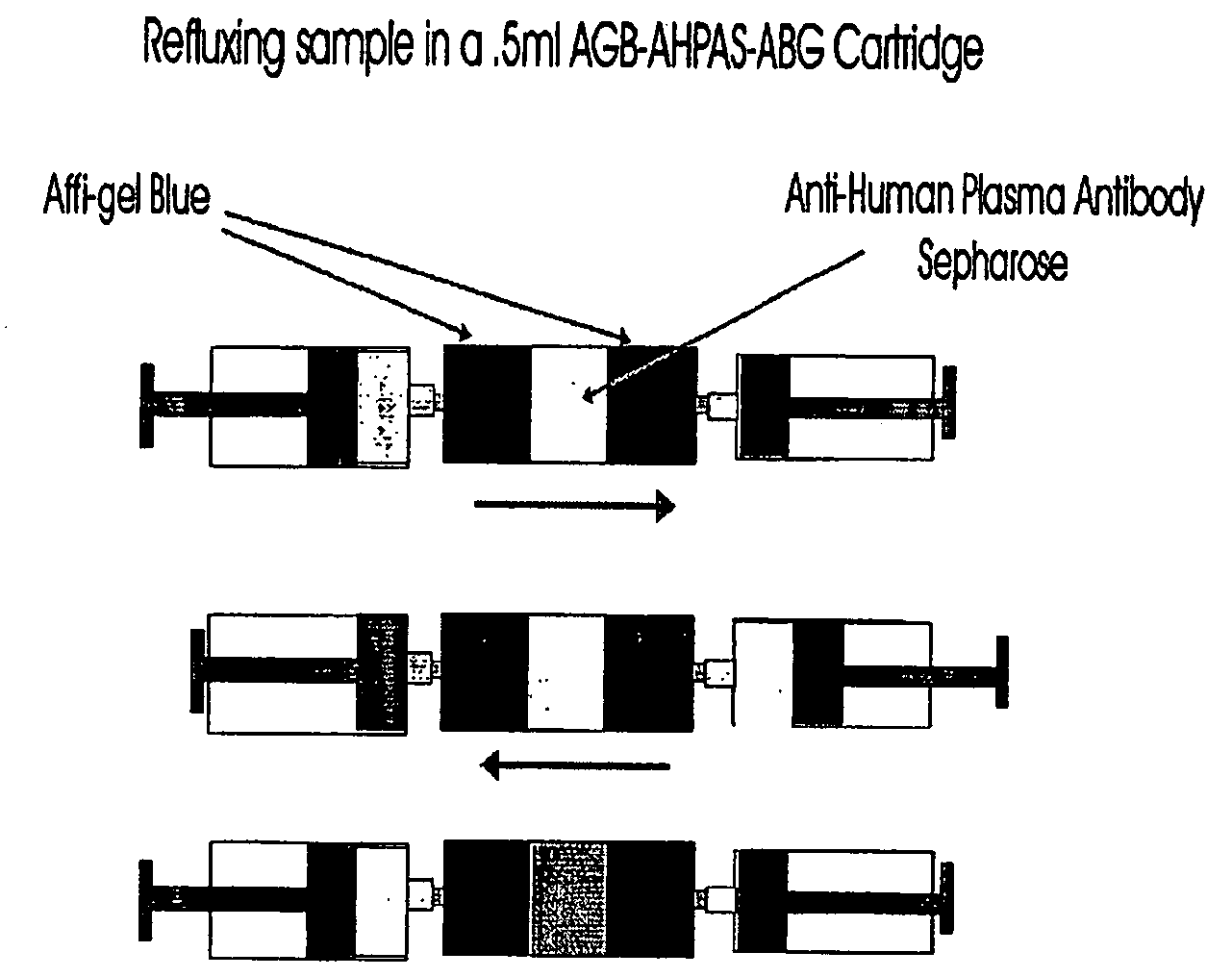
Depletion of plasma proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Polyclonal Antibodies to Human Plasma
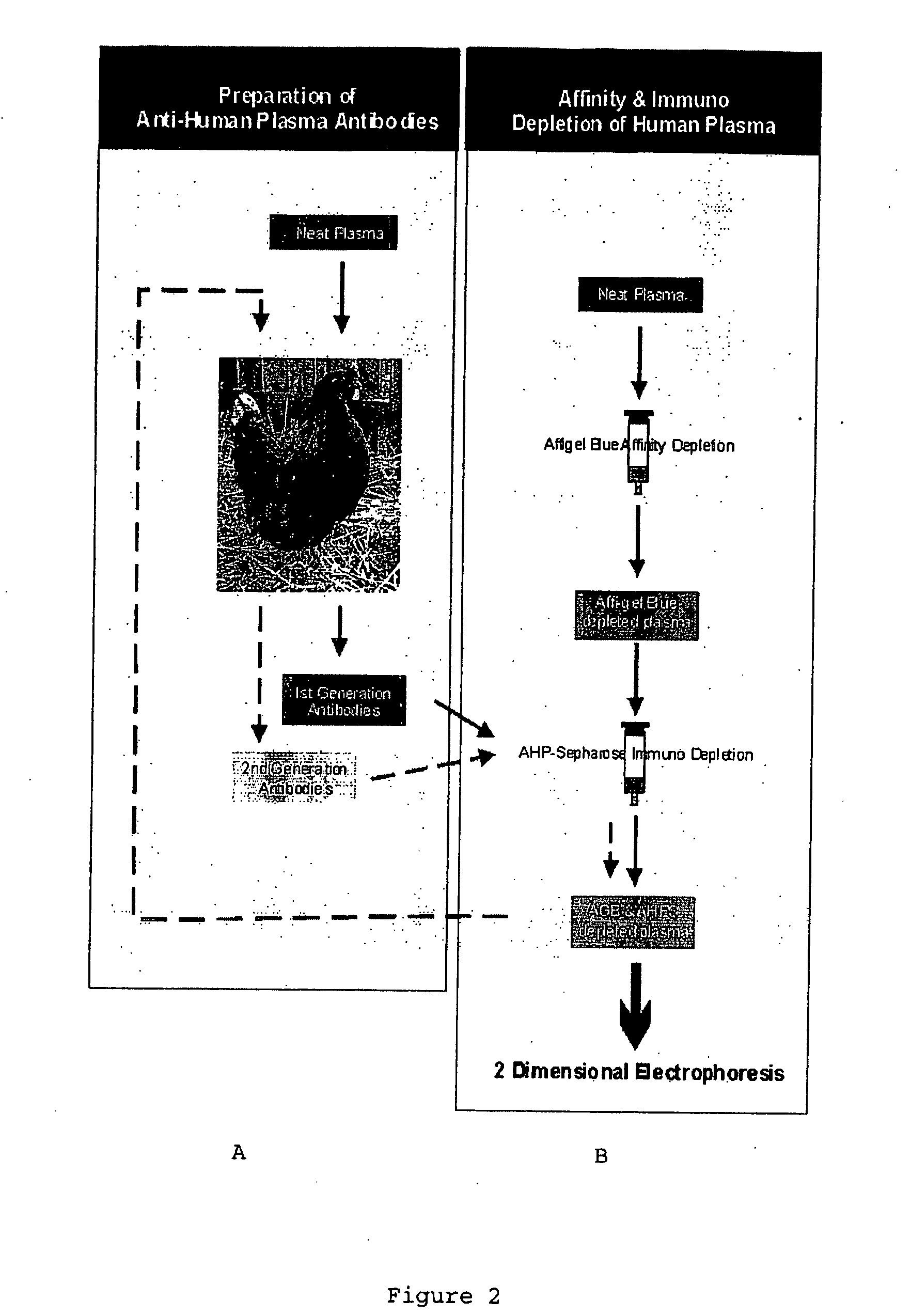
[0080]First generation polyclonal antibodies to human plasma were produced in female chickens. The procedure is summarised in FIG. 2. Chickens (14 week old White Leghorn / Rhode Island Red cross) were immunised according to the recommendations of the 21st European Centre for the Validation of Alternative Methods (ECVAM) workshop, using 1 mg plasma proteins / bird (12.5 μl of 80 mg / ml) suspended in saline (87.5 μl) / Freund's Incomplete Adjuvant (100 μl). 100 μl of total plasma proteins were injected subcutaneously over the pectoralis major muscle, using a 25-gauge needle at four sites (i.e. 50 μl per site).
[0081]Birds received three booster injections as described above, 4, 8 and 12 weeks later. Eggs were collected prior to immunization and the yolks stored at −20° C. Eggs were collected daily during the immunization schedule, up to—30 days after the last booster injection and the yolks extracted as described in Example 2.
example 2
Extraction of IgY
[0082]Egg yolks (10 per batch) were separated and then suspended in 2 volumes of 100 mM phosphate buffer (pH7.6) in a glass beaker. An equal volume of chloroform was added and then stirred for 5 min at room temperature. The resultant emulsion was then transferred to 100 ml glass centrifuge tubes and centrifuged at 2000 g for 1 h at 4° C. The supernatant was collected and its volume determined. PEG 6000 (Sigma Chemical Company, St Louis, USA) was dissolved in the supernatant to final concentration of 12% w / v, incubated for 10 min at room temperature and then centrifuged at 2000 g for 1 h at 4° C. The supernatant was discarded and the pellet resuspended in 100 mM phosphate buffer pH7.6 (⅙ original yolk volume) and stored at −20° C. as 1 ml aliquots.
[0083]Egg yolks were collected for four weeks following the final immunization, pooled and extracted as described above.
[0084]The binding characteristics of the extracted antibodies were determined by 2DE Western Blot analy...
example 3
Coupling of IgY to Sepharose 4B
[0085]PEG 6000 was dissolved in 2 ml IgY solution (17.3 mg protein / ml), incubated for 10 min at room temperature and then centrifuged at 2000 g for 1 h. The pellet was resuspended in coupling buffer (0.1M NaHCO3 pH 8.3, containing 0.5M NaCl) to a final concentration of 7.5 mg protein / ml.
[0086]CNBr-activated Sepharose 4B (Pharmacia; 1 g) was suspended in 20 ml of 1 mM HCl. The suspension was then washed with 200 ml 1 mM HCl on a sintered glass filter. The washed gel was resuspended in the IgY solution, and mixed on a rotary mixer for 18 h at 4° C. The gel was then washed with 5 volumes of coupling buffer and incubated in 0.1M Tris-HCl buffer, pH8.0 for 2 h at 4° C. The gel was washed 3 times alternately with 5 volumes 0.1M acetate buffer pH 4.0 containing 0.5M NaCl, and then 0.1M Tris HCl pH 8.0 containing 0.5M NaCl. The anti-human plasma antibody-Sepharose 4B (AHP-Sepharose) gel was then stored at 4° C. in 0.01 M phosphate-buffered saline, pH7.4, conta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com