Process for forming thin film encapsulation layers
a technology of encapsulation layer and thin film, which is applied in the direction of coating, solid-state device, chemical vapor deposition coating, etc., can solve the problems of oled materials being subject to degradation, affecting the performance of oled materials, and affecting the quality of oled materials,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Description of the Coating Apparatus
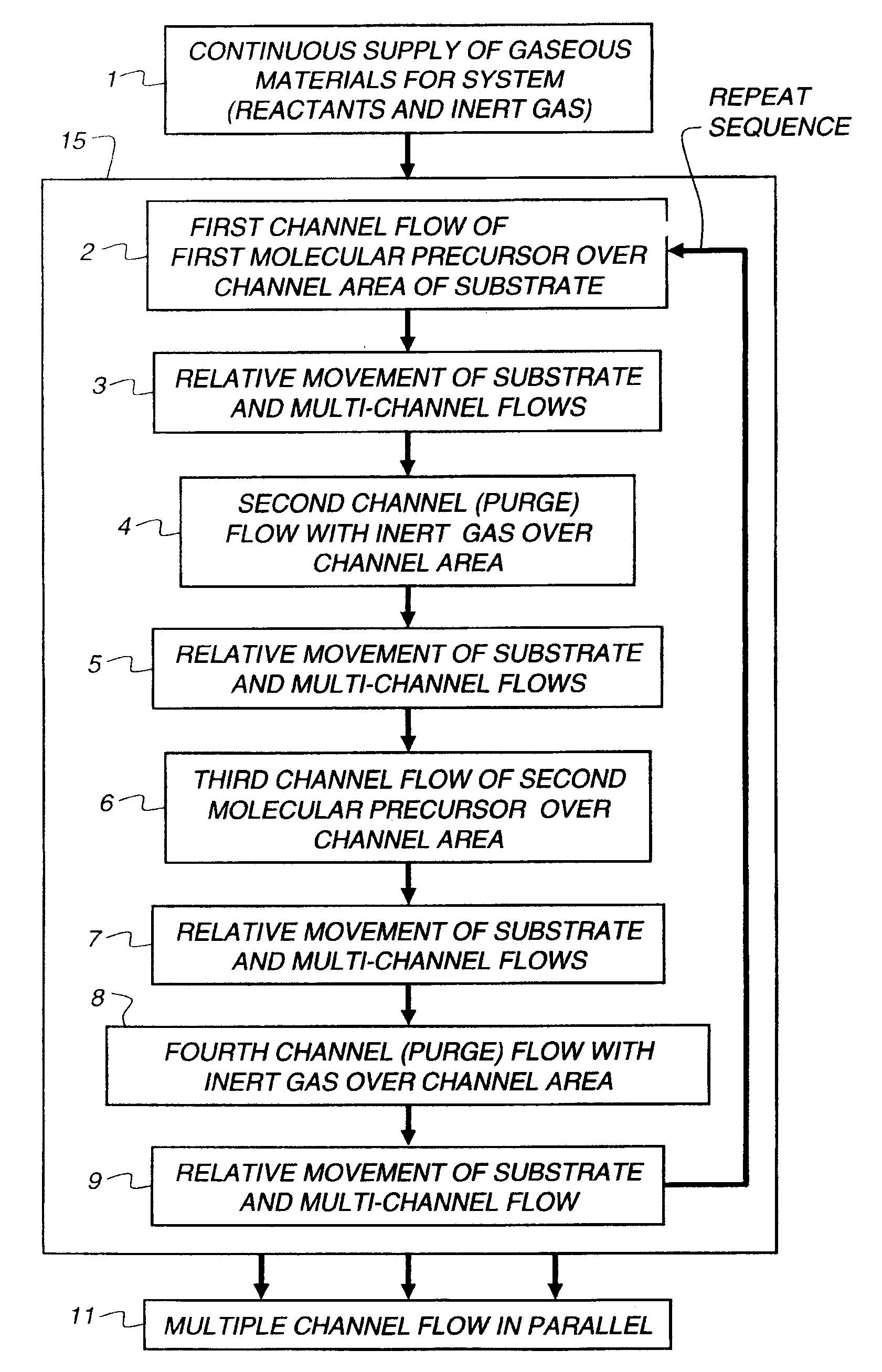
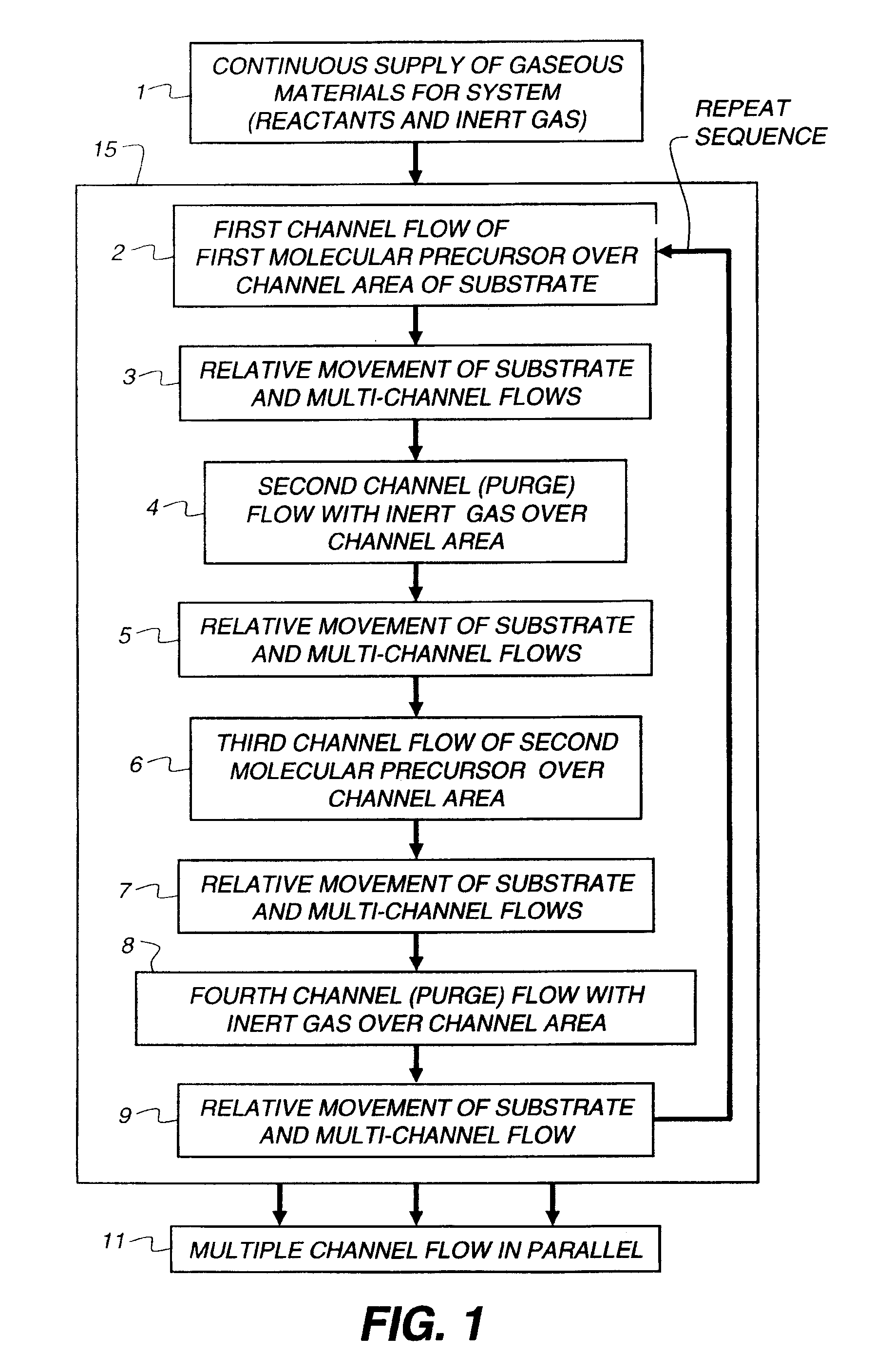
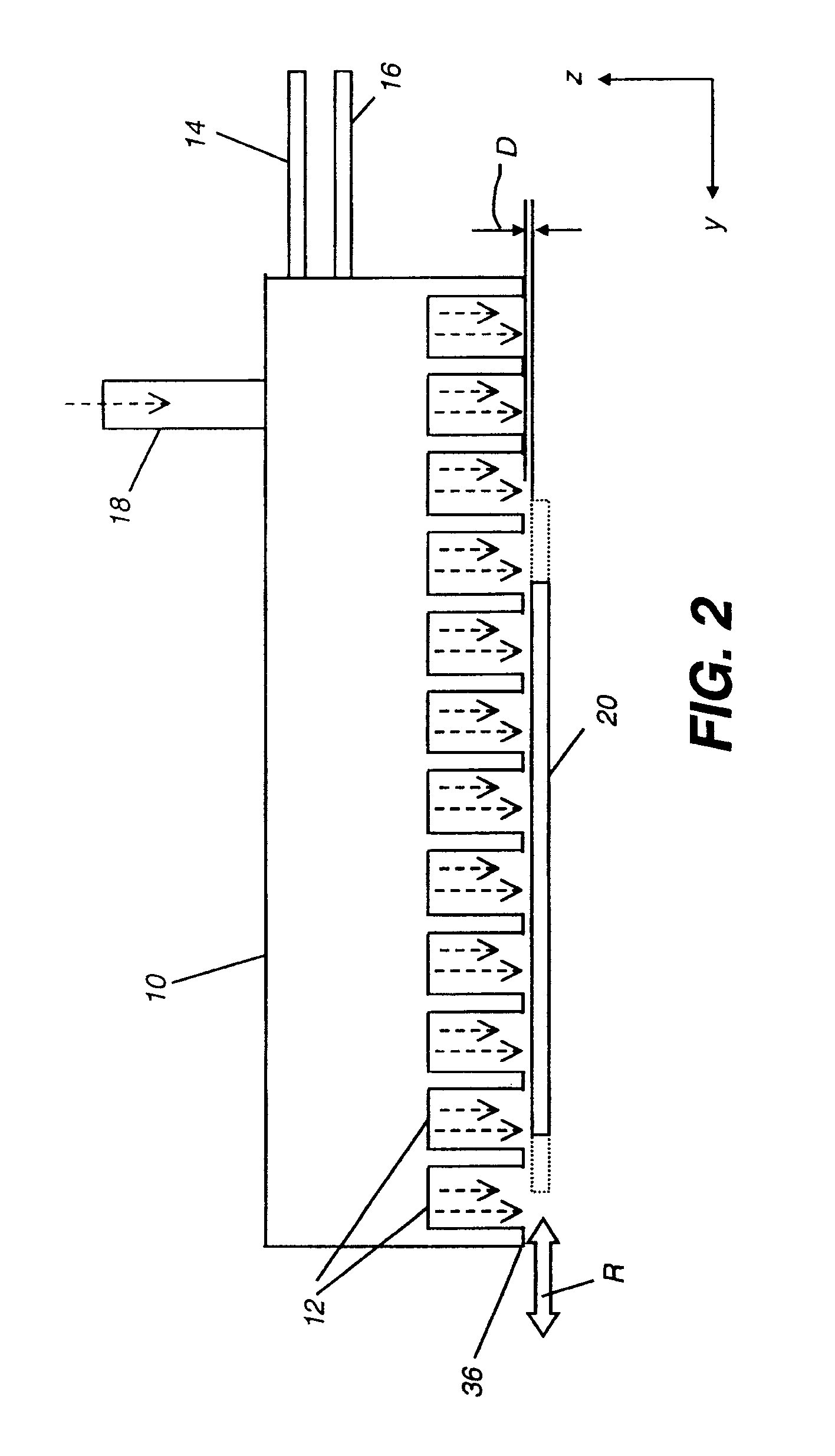
[0147]All of the following thin film examples employ a coating apparatus, for atomic layer deposition, having the flow setup indicated in FIG. 3, which is a block diagram of the source materials for a thin film deposition process according to the Examples.
[0148]The flow setup is supplied with nitrogen gas flow 81 that has been purified to remove oxygen and water contamination to below 1 ppm. The gas is diverted by a manifold to several flow meters which control flows of purge gases and of gases diverted through bubblers to select the reactive precursors. In addition to the nitrogen supply, air flow 90 is also delivered to the apparatus. The air is pretreated to remove moisture.
[0149]The following flows are delivered to the ALD coating apparatus: metal (zinc) precursor flow 92 containing metal precursors diluted in nitrogen gas; oxidizer-containing flow 93 containing non-metal precursors or oxidizers diluted in nitrogen gas; and nitrogen purge flow...
##ventive example 1
Inventive Example 1
[0182]Various multilayers of a Al2O3 / ZnO stack, wherein the number and thickness of the layers were varied were made and tested. The multilayer stacks were about 2000 Å in total thickness. The coating for these two inventive devices comprised the following combination of layers:
Al2O3120 ÅZnO100 ÅAl2O3100 ÅZnO150 ÅAl2O3200 ÅZnO200 ÅAl2O31000 Å
[0183]The results showed that the multilayered film stacks consisting of Al2O3 and ZnO layers exhibited less or no cracks, meaning that the stress was better accommodated by the multilayer film stacks.
[0184]It was also shown that the multilayered Al2O3 / ZnO film stacks can provide good protection: two of the inventive devices exhibited no dark spot growth in the center of the OLED pixels (edge growth can be eliminated by optimization of the geometry and the flow rates) after 24 and 48 hours in a humidity chamber.
##ventive example 2
Inventive Example 2
[0185]An OLED device was coated with an encapsulation film containing a mixture of Al2O3 / ZnO prepared by combining precursors for two oxides in the microchamber slots of a spatially dependent atomic layer deposition head, using water in another channel.
[0186]A total of 450 oscillation cycles of the delivery head was performed. During the coating process, a 120 Å layer of pure Al2O3 was first deposited. Then the flows of metal precursors to the trimethylaluminum bubbler flow and to the diethylzinc bubbler flow were gradually modified to increase the relative amount of ZnO and decrease the relative amount of Al2O3 until the film reached 100% of ZnO. Then the process was repeated in the opposite direction, diminishing the relative amount of ZnO while increasing the relative amount of Al2O3 such that the final 100 Å of material consisted of Al2O3 only. The total thickness of the mixed Al2O3 / ZnO film was approximately 2000 Å.
[0187]After the coating process was complete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| speed | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com