Novel non-selective cation channel in neuronal cells and method for treating brain swelling
a cation channel and neuronal cell technology, applied in the field of cell biology, neurophysiology, medicine, can solve the problems of serious consequences of nerve injury, morbidity and mortality, worsening outcome, etc., and achieve the effect of increasing the risk of stroke for subjects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Morphological Changes with ATP Depletion Using NaN3
[0396]Cultured neural cells have been shown to swell upon ATP depletion. See, Jurkowitz-Alexander et al., 1992; Jurkowitz-Alexander et al., 1993. Freshly isolated NRAs depleted of ATP also results in cell swelling. Ischemia or traumatic injury in brain also causes depletion of ATP in brain neural cells.
[0397]The surfaces of freshly isolated NRAs are highly complex, exhibiting small membrane evaginations and fine processes that decorate the entire cell surface, as shown in the scanning electron micrograph in FIG. 14A. Exposure of NRAs to NaN3 (1 mM) causes changes in the surface appearance, characterized early-on by loss of complex structure and development of surface blebs (FIG. 14B), followed later by a grossly swollen appearance with complete loss of fine structure and formation of multiple large blebs (FIG. 14C). Therefore, NRAs undergo blebbing and swelling after NaN3— induced ATP depletion.
[0398]Phase contrast microscopy is al...
example 2
General Electrophysiological Properties of NRAs
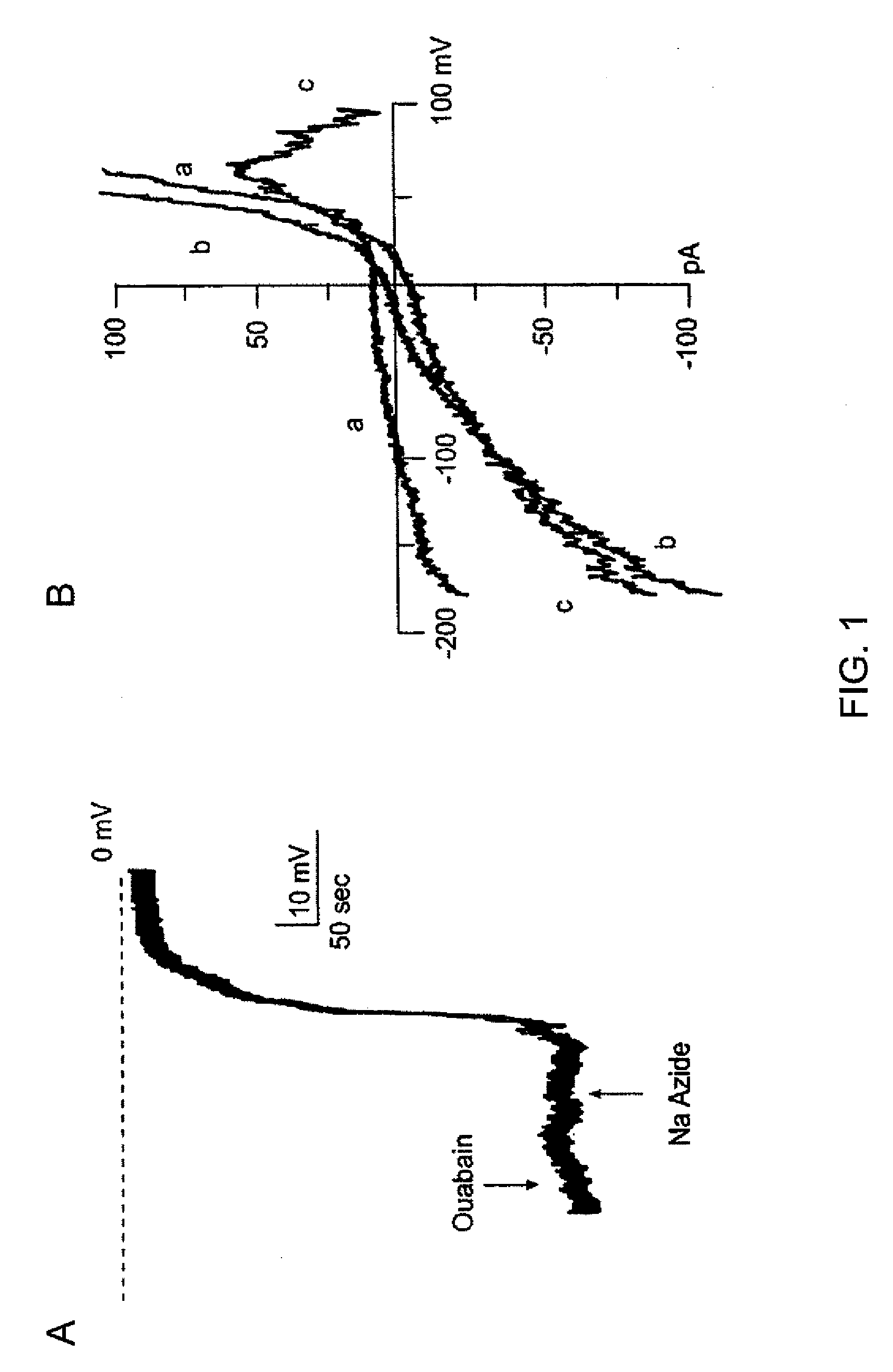
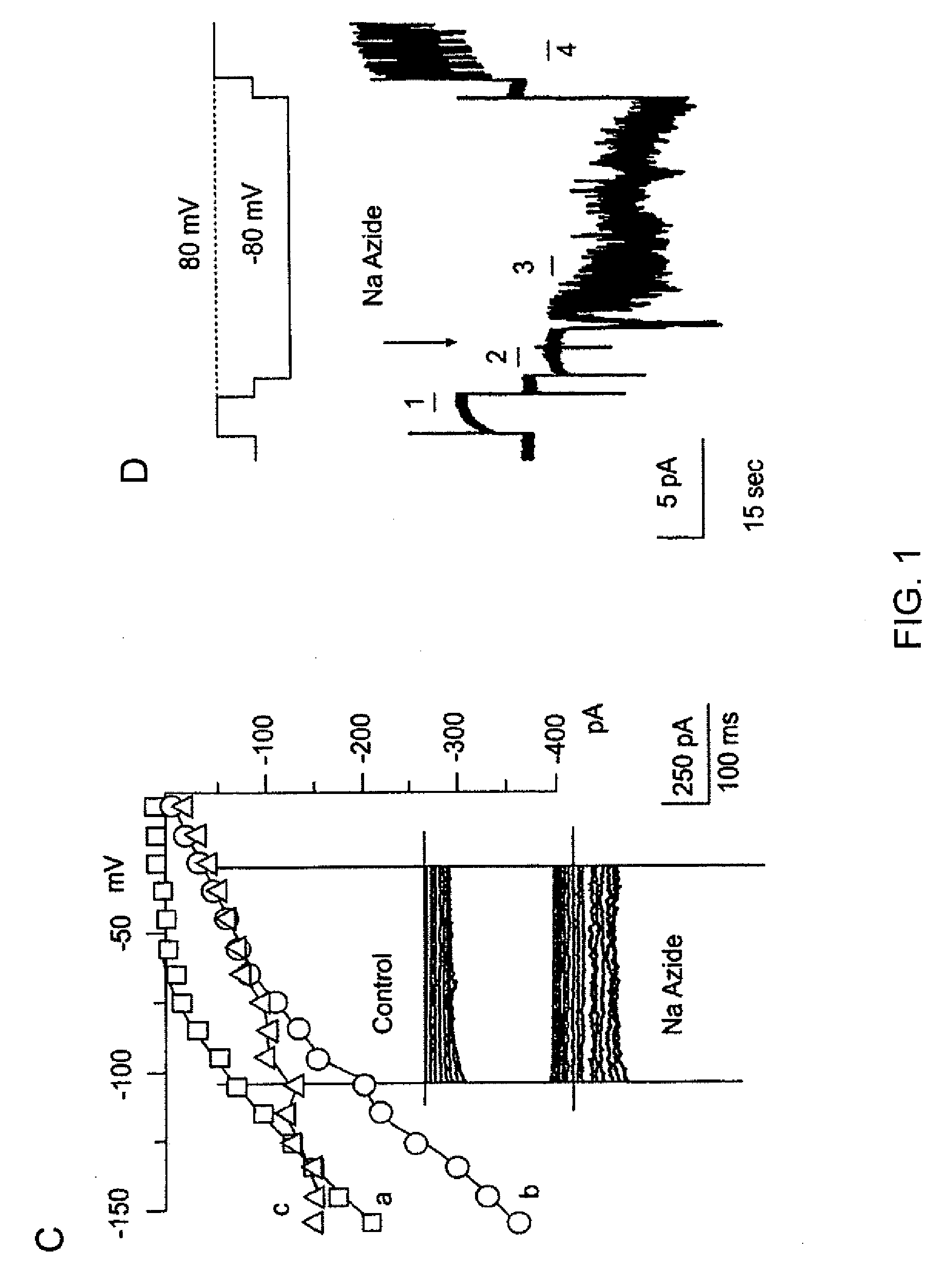
[0400]The macroscopic currents of whole cell preparations of N u s are characterized by small inward currents at negative potentials, large outward currents at positive potentials, and a flat “plateau” region at intermediate potentials. NRAs exhibit macroscopic currents that are consistent with observations in primary cultured cells of the same origin. See, Chen et al., 2003; Chen et al., 2001. The NRAs exhibited inward currents negative to the K+ equilibrium potential (EK) are usually 2+-activated K+ channel. See, Perillan et al., 1999. The outward current that remains in the presence of charybdotoxin can be further blocked by 4-aminopyridine (5 mM), and exhibits kinetic properties typical of a delayed rectifier K+ channel. Consistent with a previous report (Perillan et al., 1999), fast inward voltage dependent currents attributable to Na+ channels are observed in less that 1% of NRAS.
NaN3 Elicits Depolarizing Inward Current Due to 35 ...
example 3
Relative Permeabilities and Pore-Size
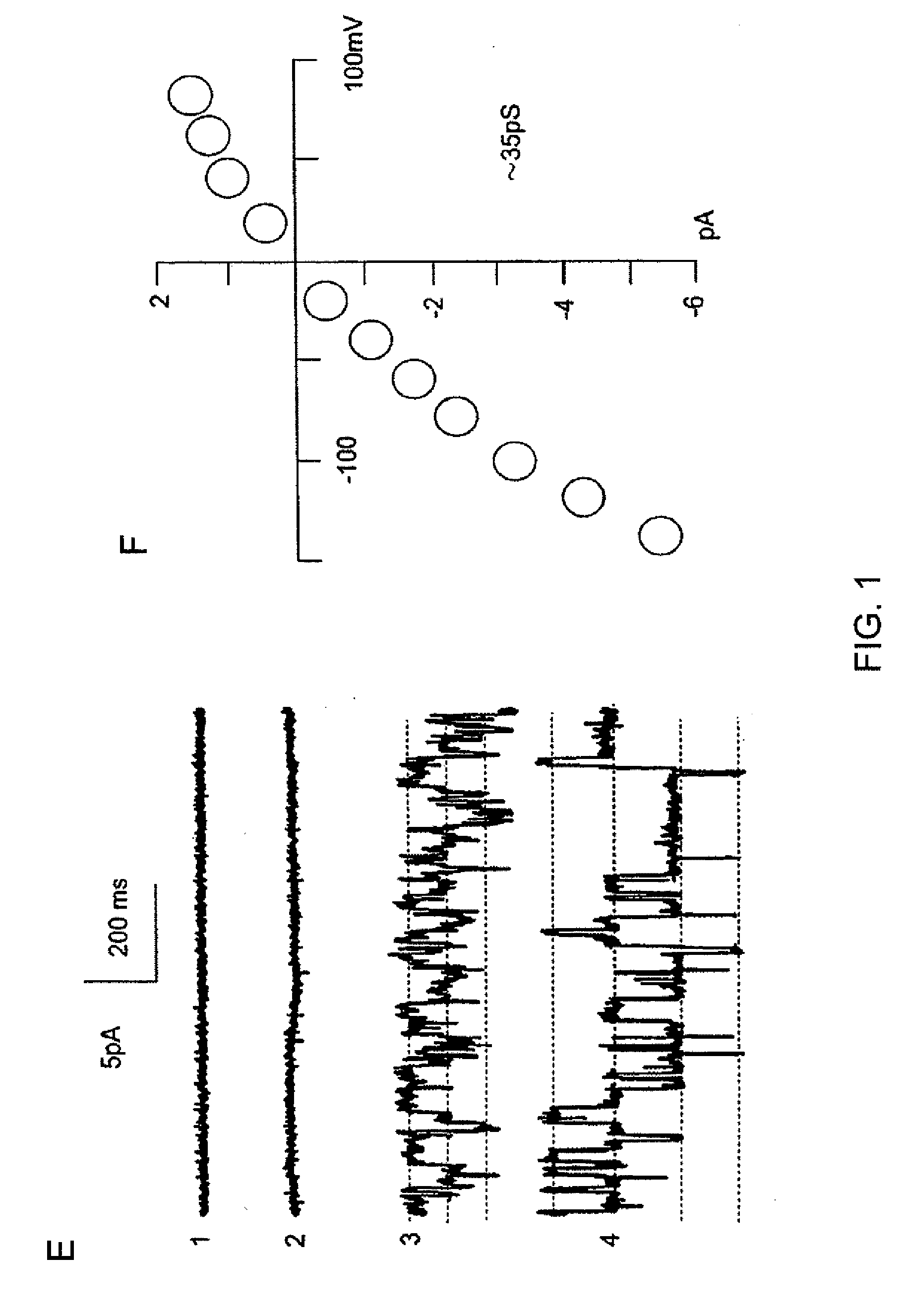
[0409]The channel is further characterized using membrane patches in the inside-out configuration. Records obtained during test pulses to various potentials with equal [K+] on both sides of the membrane are shown in FIG. 2A. Amplitude histograms are constructed of events observed at potentials from −140 mV to +100 mV, and values (mean±SE) for 4 patches are plotted and show in FIG. 2B. Fit of the data to a linear equation indicates a slope conductance of 35 pS, with an extrapolated reversal potential (Erev) of +0.1 mV, close to the expected K+ reversal potential (EK) of 0 mV.
[0410]In addition to conducting K+, the channel transports a variety of alkaline ions (FIG. 3A), indicating that it is a non-selective cation channel. In inside-out patches, the conductance of the channel is measured with various alkaline ions in the pipette solution, including Cs+, Na+, Rb+, K+, and Li+, always with equimolar K+ in the bath solution. Current-voltage data are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| single-channel conductances | aaaaa | aaaaa |
| single-channel conductance | aaaaa | aaaaa |
| single-channel conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com