Diseases of the cardiovascular
system affect millions of people each year and are a leading
cause of death throughout the world.
The cost to society from such diseases is enormous both in terms of the number of lives lost as well as in terms of the costs associated with treating patients through traditional surgical techniques.
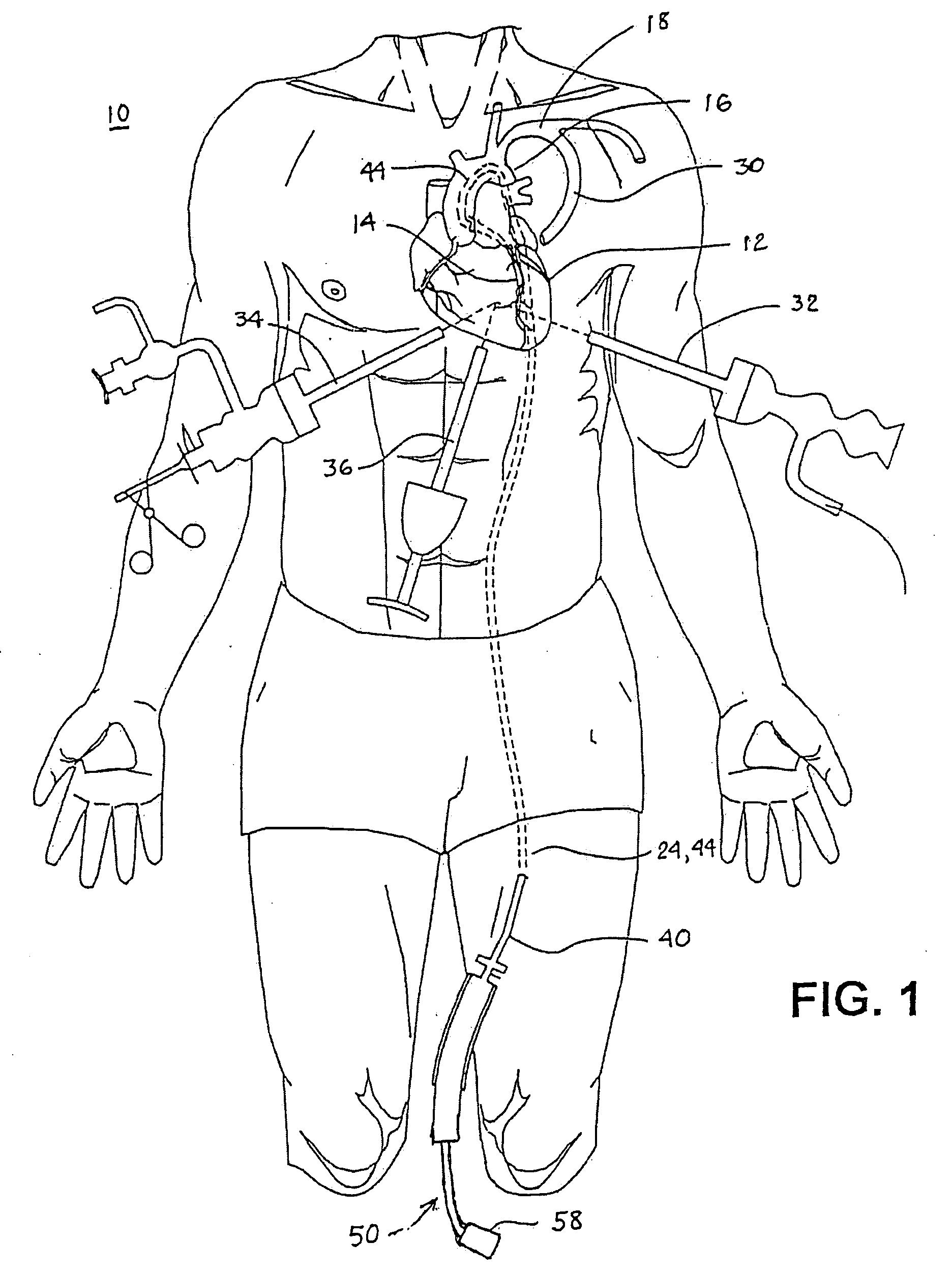
The
trunk hosts a number of potential grafts including, the left internal mammary
artery (left IMA), the right internal mammary artery (right IMA), the radial arteries and three visceral arteries, one in the
abdomen, and two in the lower
abdominal wall, though the latter can be quite short and are generally of limited usefulness.
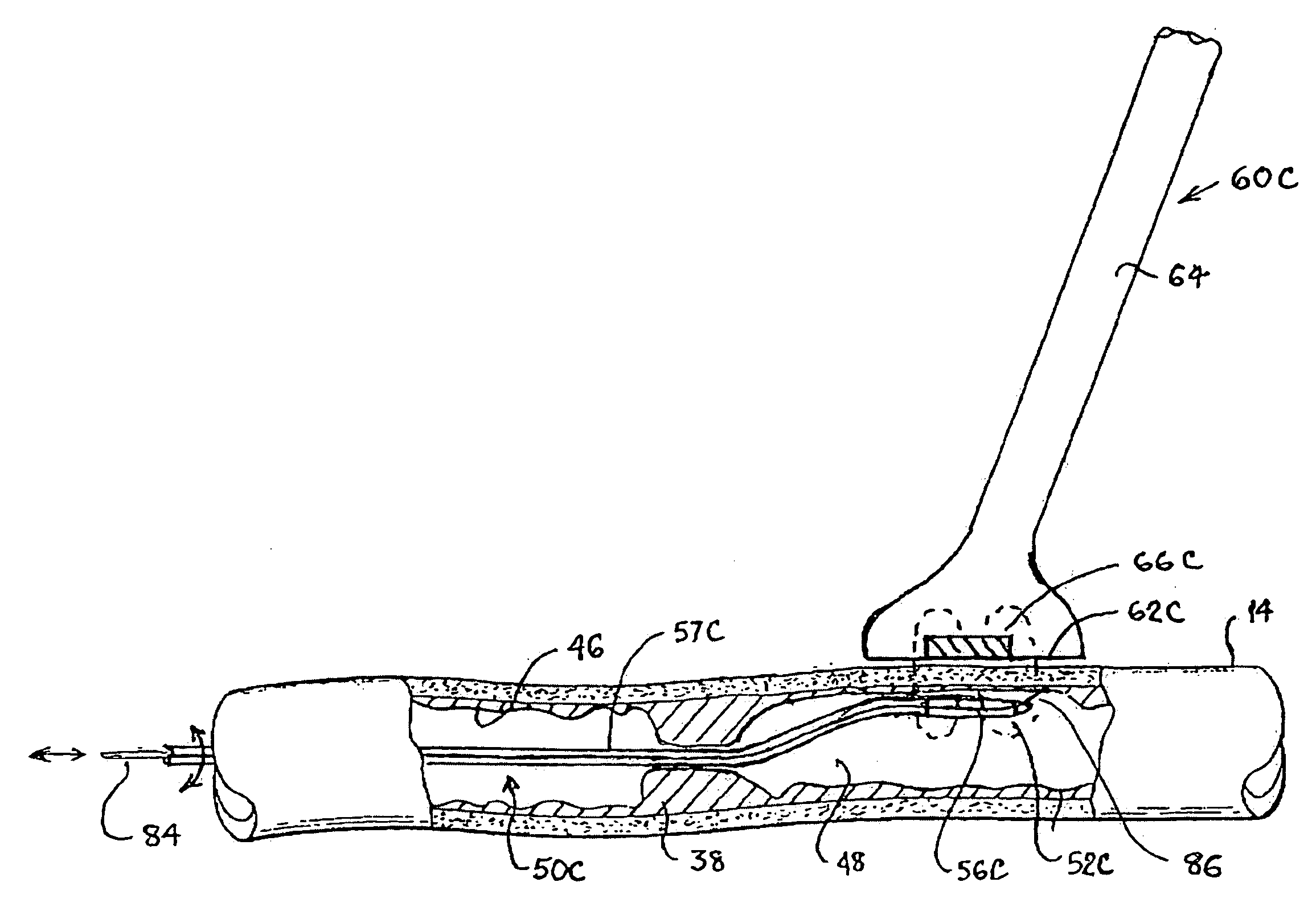
Intimal damage to both the graft and the
target vessel can also occur during delivery of the device.
In addition, the size of the device is strongly related to the size of the vessels.
Problems associated with construction of an
anastomosis using a two component intra-luminal mechanical
coupling device can include mounting of the vessels and connection of the components.
However, mounting of the graft to the
coupling device may not be easy.
Damage can occur to the intimal layer when everting the graft onto the device for two reasons: 1) one tip of a pair of pincers used to solidly grab vessel wall to evert an artery will roughly touch and pinch the intima of the artery; and, 2) eversion causes
high strain (stretching) that can damage the
arterial wall.
In addition, care must be taken to avoid compression of tissue by the
coupling device since compression can cause pressure
necrosis.
Problems associated with construction of an
anastomosis using a two component extra-luminal mechanical coupling device also include mounting of the vessels and connection of the components.
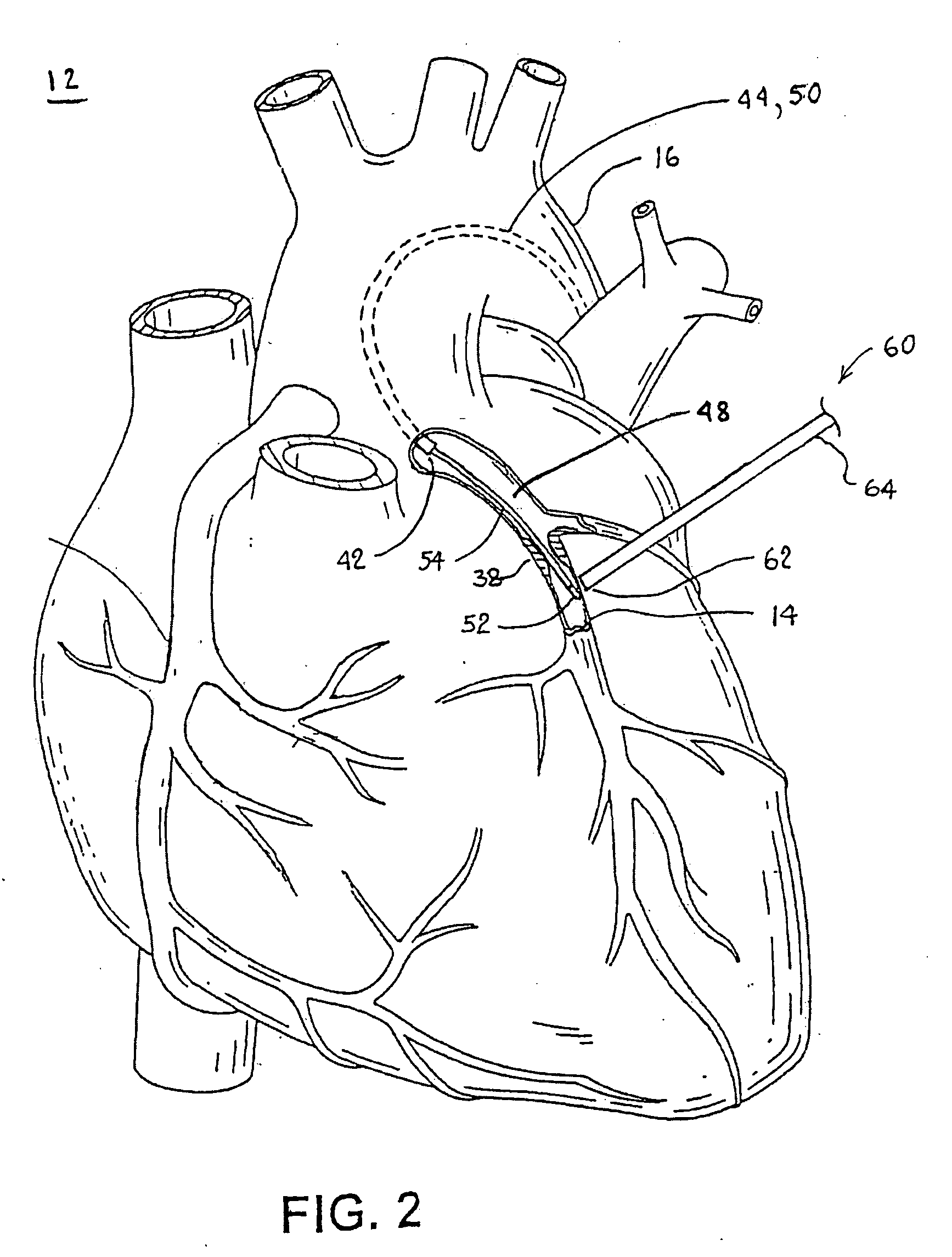
Typically, fat
layers that make it difficult to see either the artery or the
occlusion cover the epicardial surface and the obstructed cardiac artery.
However, it is difficult to accurately determine the bounds of a soft or “cheesy” occlusion, and mistakes can happen.
As a result, these operations typically require large numbers of sutures or staples to close the incision and 5 to 10 wire hooks to keep the severed sternum together.
Such
surgery often carries additional complications such as
instability of the sternum, post-operative bleeding, and mediastinal infection.
The thoracic
muscle and ribs are also severely traumatized, and the healing process results in an unattractive scar.
In this case, it is not possible for the physician to manually palpate the fatty tissue overlying the artery and occlusion to accomplish this.
However,
biplane fluoroscopy is costly and slow, and erroneous interpretation of the images often occurs.
In fact, it can be difficult to distinguish between a lumen of an artery and that of a
vein.
Smaller vessel diameters can also be harder to identify than larger
diameter vessels.
It appears the probe would be unable to precisely identify the targeted coronary artery.
Further, in a closed-chest procedure, it can sometimes be very difficult to identify a specific area of the heart while viewing the heart with an
endoscope having a limited
field of view thereby making it difficult to precisely locate the targeted vessel.
However, these transmitters and receivers will be limited in size required to fit in a source vessel or a
target vessel.
The smaller size may limit the effectiveness of the transmitters and receivers.
For example, the size of an
ultrasound transducer can limit the distance and resolution that images may be acquired.
If the physician chooses the wrong length, the source vessel may end up to short or to long, both of which may be detrimental to the creation of the anastomosis.
Again, the physician may mistakenly locate and open the wrong vasculature lumen unless the physician knows approximately where the target coronary artery resides, and it may be difficult to distinguish between a lumen of an artery and that of a
vein.
Smaller
diameter vessels may also be harder to identify than larger
diameter vessels.
Therefore, the imaging locator alone may be unable to precisely identify the targeted coronary artery.
Furthermore, in a closed-chest procedure it can sometimes be very difficult to identify a specific area of the heart while viewing the heart with an
endoscope having a limited
field of view thereby making it difficult to precisely locate the targeted vessel.
However, the ability of a surgeon to visually ascertain changes in light brightness of a light shining through a vessel wall either directly or through a
thoracoscope can be hampered if the light is diffused in the tissue of the vessel wall or is blocked by an obstruction or any fatty tissue overlying the
blood vessel and epicardium.
Moreover, environmental conditions of the operating room, particularly the brightness of the room or the surgical field, can diminish the brightness of the
transmitted light and make it difficult to see.
While these approaches may hold promise, in some instances they are either unduly complicated to practice or not specific enough.
 Login to View More
Login to View More  Login to View More
Login to View More