Transgenic avian which has foreign gene containing sequence encoding feline-derived protein and method for production thereof
a technology a production method, which is applied in the field of transgenic birds, can solve the problems of difficult to say that what was possible with a human-derived protein is also possible, and difficulty in obtaining a high level of expression of a foreign gene in animal cells,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
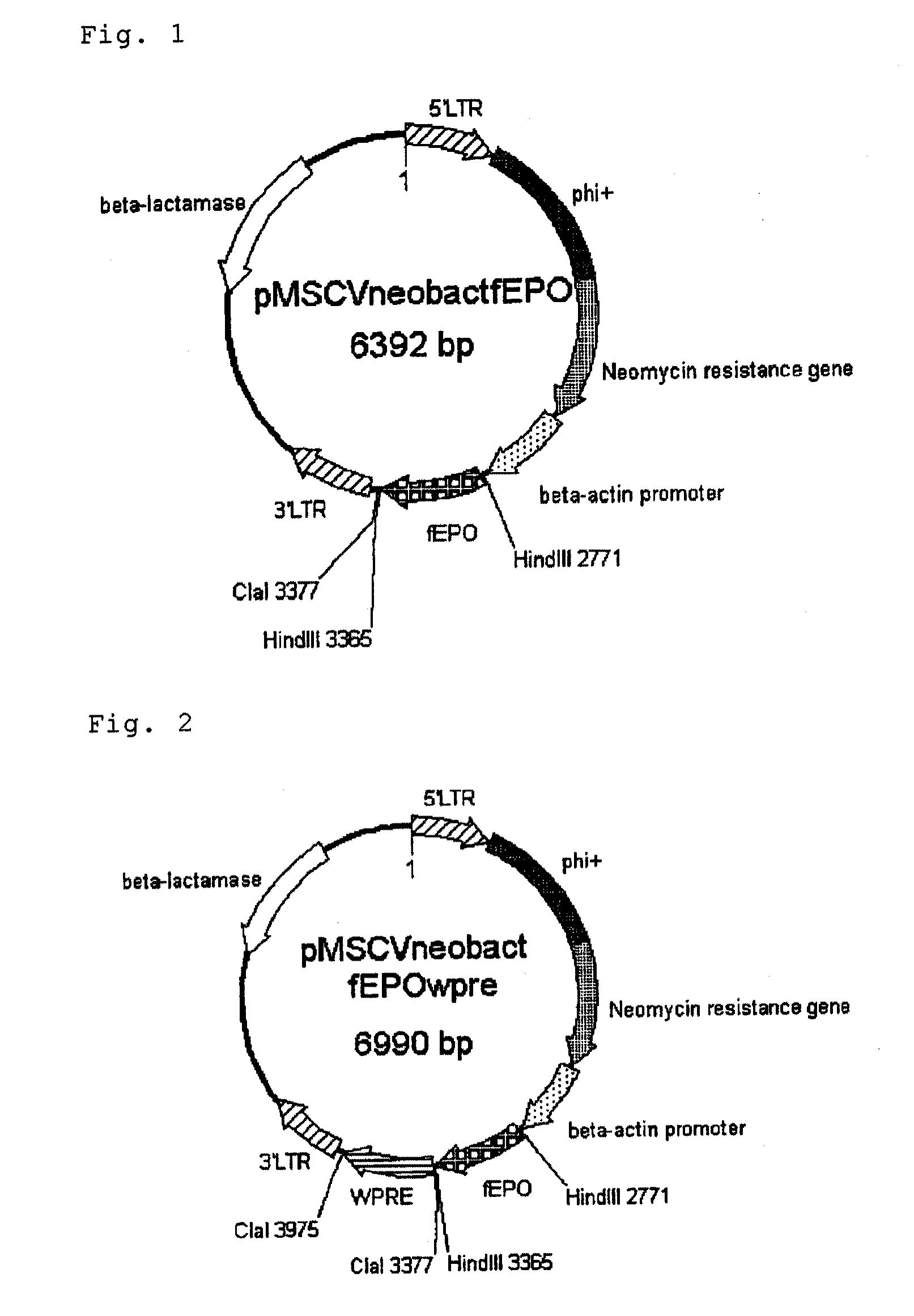
Construction of feline-derived erythropoietin gene expression plasmid pMSCVneobactfEPO
[0098]pMSCVneobact (SEQ ID NO:2) was totally synthesized based on the relevant prior art document (Gene Ther. 1994, Mar.: 1(2):136-8) and internet information (http: / / www.ncbi.nlm.NIH.gov / etc.) and inserted into pUC19 (GenBank Accession No. X02514) at a site between EheI (235) and PvuII (628) (products of Toyobo). The product was cleaved with HindIII (product of Takara Bio) and, after treatment with Alkaline Phosphatase BAP (product of Takara Bio), the desired fragment was purified and recovered using MinElute Reaction Cleanup Kit (product of QIAGEN). This was electrophoresed on 1% agarose and the desired fragment was purified and recovered using MinElute Gel Extraction Kit (product of QIAGEN) (Example 1 vector fragment).
[0099]pUCfEPO (SEQ ID NO:3) encodes, at 911 to 1489 bp, the feline-derived erythropoietin sequence. A fragment amplified by PCR using Pyrobest DNA polymerase (product of Takara Bi...
example 2
Retrovirus Vector Preparation Using pMSCVneobactfEPO and pVSV-G
[0102]Hereinafter, unless otherwise specified, the medium used was Dulbecco's Modified Eagle Medium (DMEM) (product of Gibco) containing 10% of fetal bovine serum (FBS) and 50 units / ml each of penicillin and streptomycin. The cultivation was carried out at 37° C. in the presence of 5% CO2. The plasmid DNA used in the retrovirus vector was Endo Free Plasmid Maxi Kit (product of QIAGEN).
[0103]For retrovirus vector preparation from the plasmid pMSCVneobactfEPO constructed in Example 1, a collagen-coated culture dish having a diameter of 100 mm was sowed with GP293 packaging cells having the gag and pol genes (5×106 cells / dish; 70% confluent) (90% confluent on the next day). On the next day, the medium was removed, and 7.2 ml of the medium and 10 μl of 25 mM chloroquine (product of Sigma) were added, followed by further 1 hour of cultivation. A 56-μl portion of Lipofectamine 2000 (product of Invitrogen) was suspended in 1.4 ...
example 3
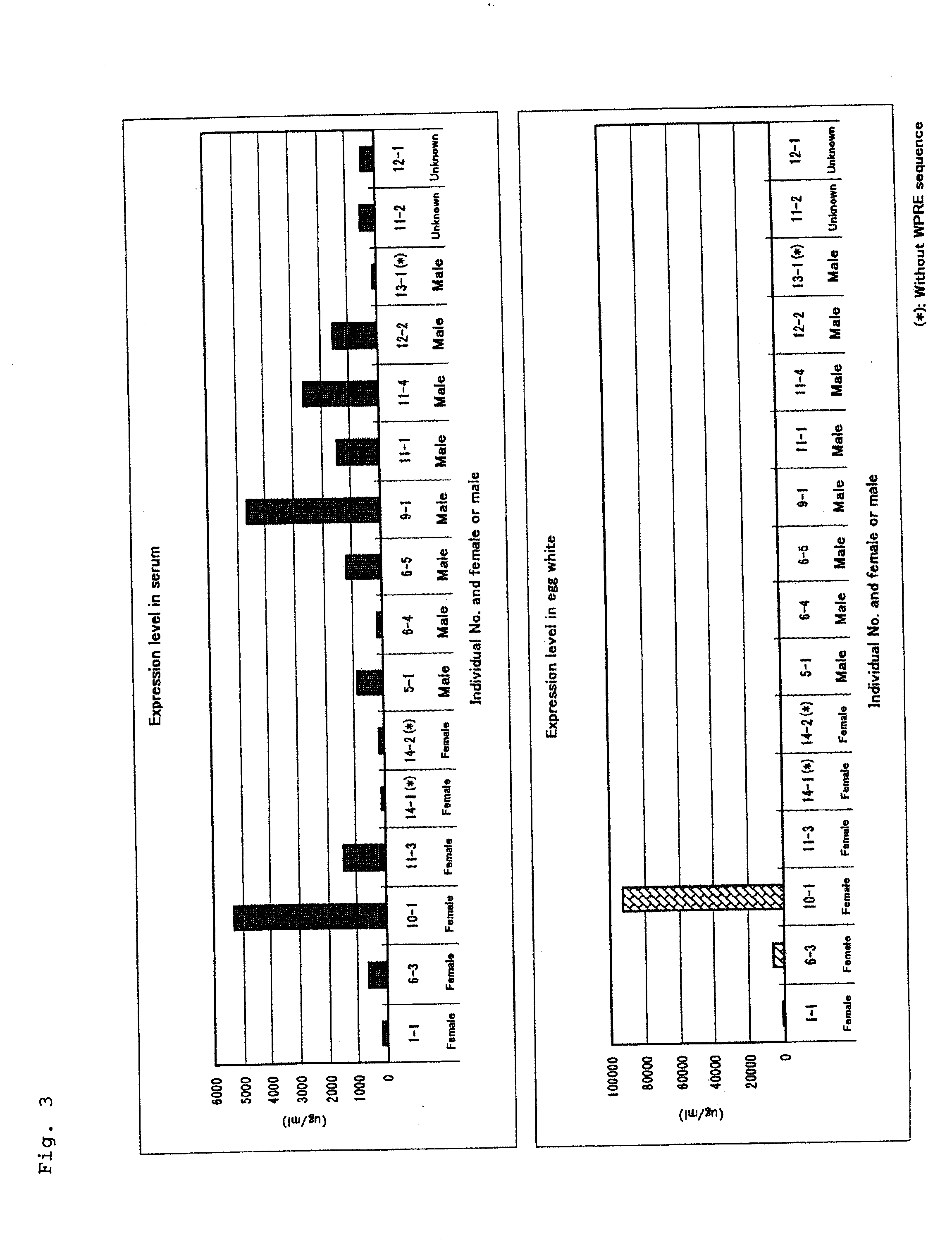
[0105]The virus titer is defined as the number of infected cells after addition of the virus-containing fluid to NIH3T3 cells (American Type Culture Collection CRL-1658). A 1-ml portion of the virus solution of Example 2 as diluted at a dilution ratio of 102 to 106 was added to 5×104 NIH3T3 cells contained in each well (base area about 9.4 cm2) of each 6-well culture plate, and the proportion of cells expressing the neomycin resistance gene as a marker was determined based on the resistance to G418. If 4 colonies appear at a dilution ratio of 106, the virus titer will be 4×106 cfu / ml.
[0106]More specifically, 6-well culture plates were sowed with 5×104 NIH3T3 cells per well on the day before the start of titer measurement, and the cells were cultured. On the next day, the cell culture medium was replaced with 900 μl of the medium containing 9 μg / ml of polybrene, the virus-containing fluid was diluted to 10−1 to 10−5 with the medium and 100-μl portions of each d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight average molecular weight | aaaaa | aaaaa |
| weight average molecular weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com