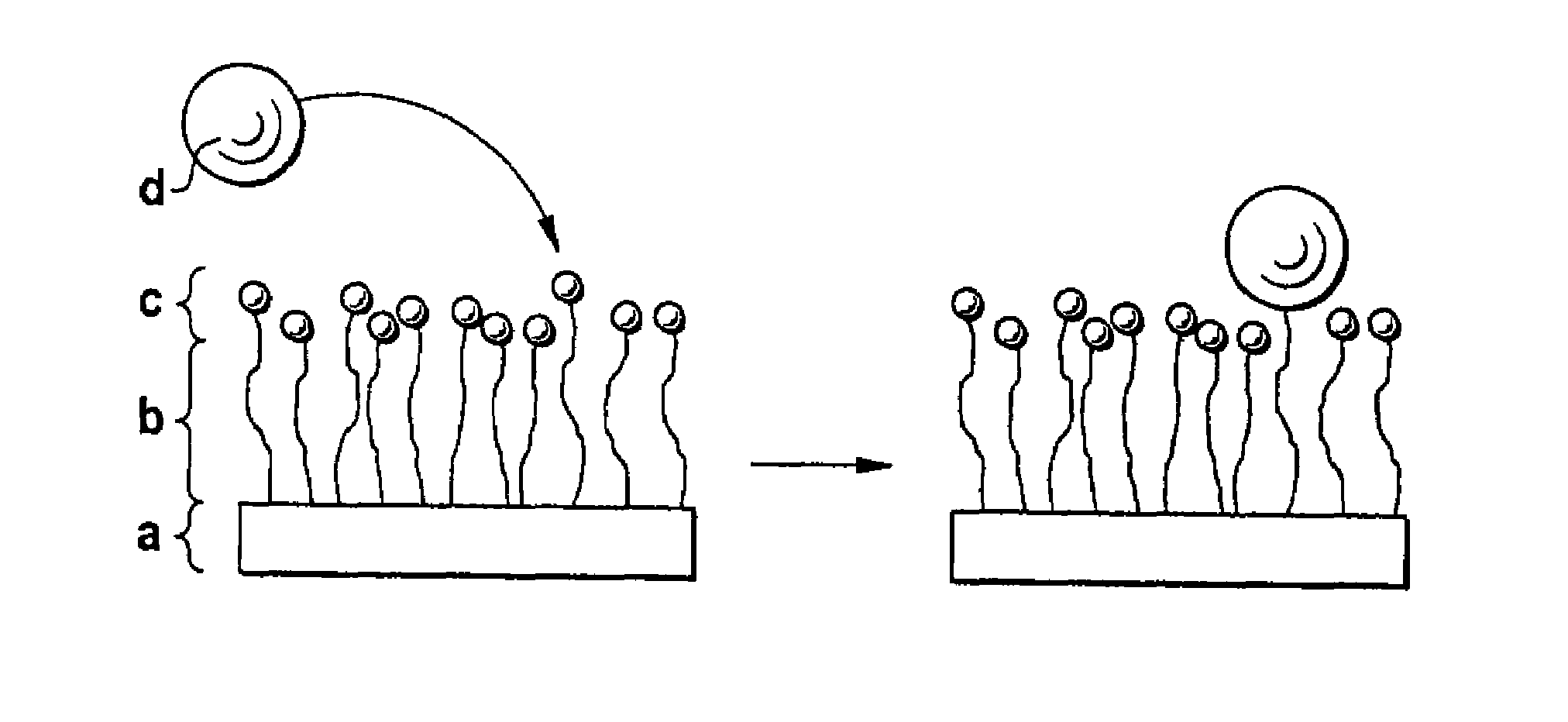
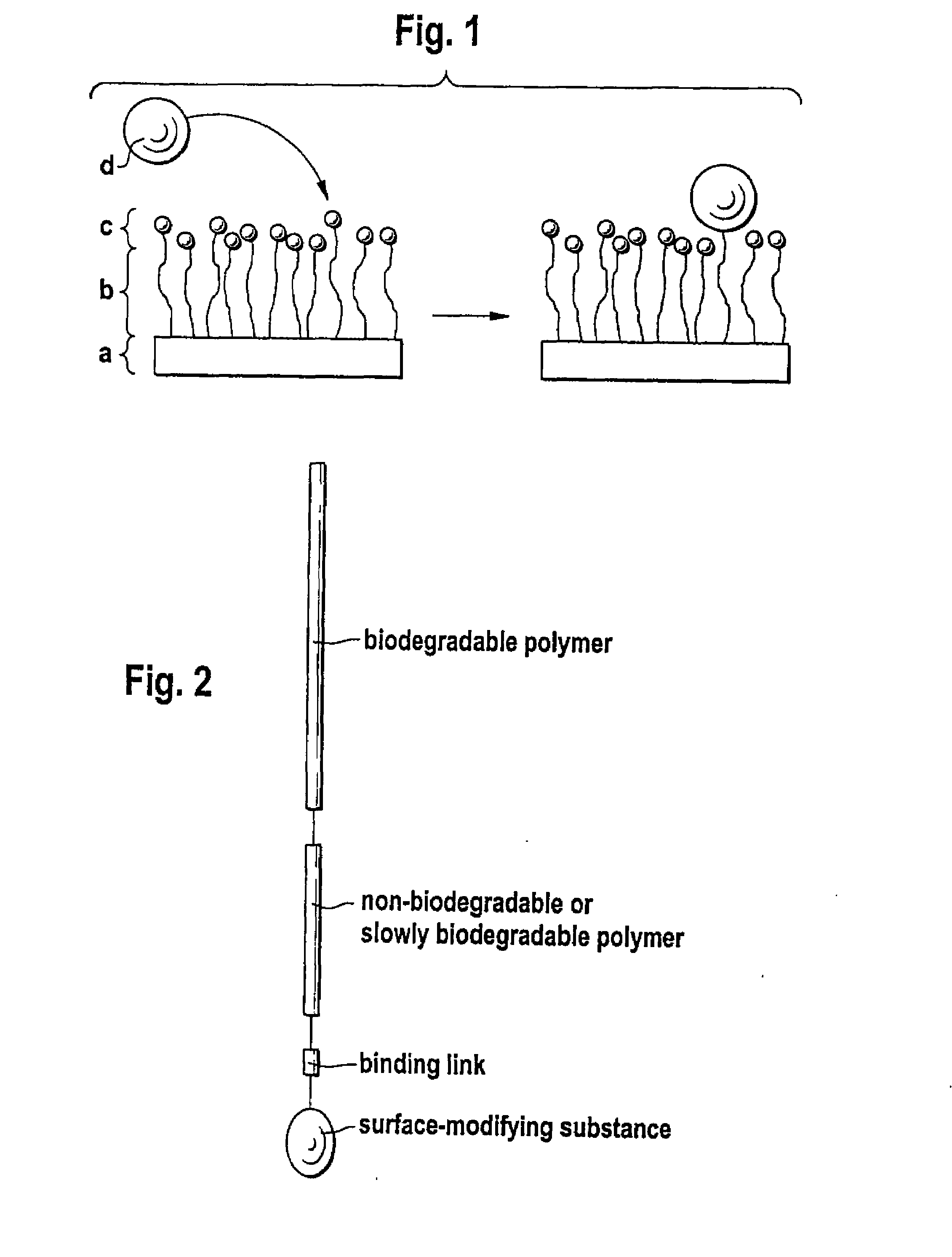
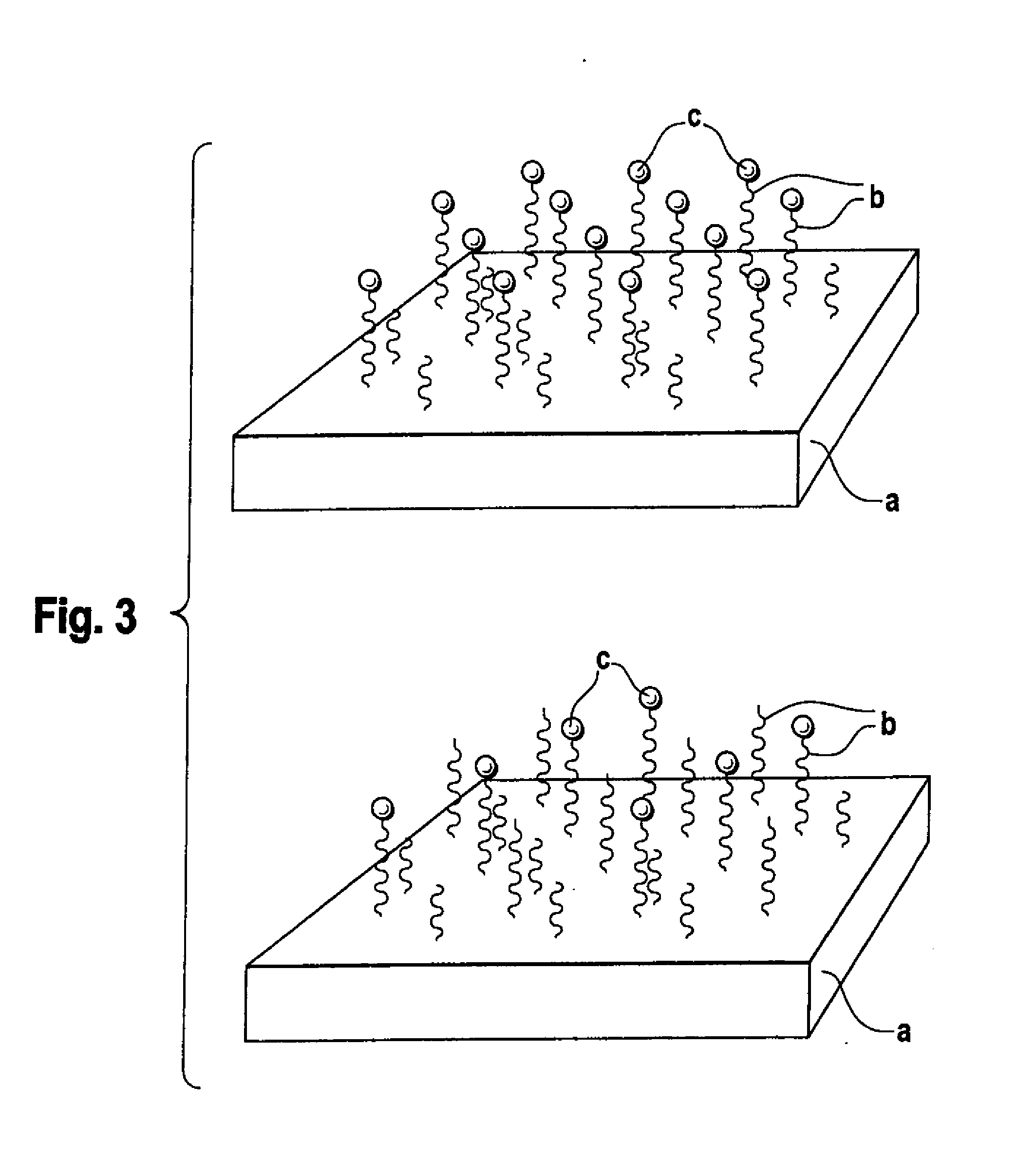
Biodegradable block copolymers with modifiable surface
a biodegradable and biocompatible technology, applied in combinational chemistry, pharmaceutical non-active ingredients, chemical libraries, etc., can solve the problems of biodegradable medicinal agent carrier not releasing dosage to the desired extent, not with the desired kinetics, and reducing so as to increase the period of degradation, promote the formation of micelles, and increase the mechanical strength of polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
Example 1
Production of NH2-PEG-PLA
[0164]a) Synthesis of NH2-PEG. Production was conducted in accordance with Yokohama, M. et al. Bioconj. Chem. 3 (1992) 275-276.
[0165]The desired amount of ethylene oxide was passed into dry THF in a three-necked flask at −79° C. (dry ice+methanol bath) and dissolved therein. The ethylene oxide bottle was weighed after introduction, and thus the presented amount of ethylene oxide was determined. In accordance with the desired molecular weight of the polymer, the calculated amount of 0.5M solution of potassium-bis-(trimethylsilyl) amide in toluene was then added from a dropping funnel.
[0166]The reaction mixture was then stirred in the closed three-necked flask at 20° C. for 36 hours. The polymer solution thus obtained was dropped into the 12-fold amount of ether, and the precipitated polymer was filtered out. After the polymer obtained was dissolved in THF, a small amount of 0.1N hydrochloric acid was added and the silylamide was thus split. The solut...
example 2
Production of Amino-polyethylene Glycol-poly-L-lactide (NH2-PEG-PLLA)
[0172]The procedure was essentially as in Example 1b). However, cyclic L-dilactide was used instead of the cyclic D,L-dilactide. Further, after rotation three times with dichloromethane, the polymer obtained was once again dissolved in dichloromethane and dropped into ice-cooled diethylether. The polymer thread thus obtained were separated through a filter and passed into a vacuum drying cupboard for drying.
[0173]Determination of the molecular weight was achieved by GC and determination of the numerical mean molecular weight was also achieved by 1H-NMR via calculation of the integrals.
example 3
Linkage of Surface-modifying Substances d)
[0174]Binding of surface-modifying substances can be conducted in accordance with the processes described in Hill, M. et al. FEBS Lett. 102 (1979) 282-286; Schulman, L. H. et al. Nucleic Acids Res. 9 (1981) 1203-1217.
[0175]The linkage of surface-modifying substances d) to the block copolymer according to the disclosure obtained in accordance with Example 1 can occur in two ways, in principle. Firstly, it is possible to bind the substance d) and the block copolymer in solution if the substance d) passes through the subsequent processing steps undamaged. Alternatively, the block copolymer may firstly be processed to the desired form and the substance d) is then linked. In both cases, it should be assured by buffering that an amino group, for example, is present in unprotonated form in order to obtain quantitative yields where possible. Moreover, with buffering the location of the bond to the substance d) can still be controlled if the pH is se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com