Method for producing an acidic substance having a carboxyl group
a technology of carboxyl group and acidic substance, which is applied in the direction of bacteria peptides, peptide sources, peptide sources, etc., can solve the problems of increasing the cost of culture, increasing the cost of isolating or purifying the product, and unable to produce acidic substances, so as to achieve efficient production of acidic substances and acidic substances.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
Construction of Pantoea ananatis Strain Resistant to λ Red gene Product
[0204]In order to disrupt the sdhA gene in Pantoea ananatis, a recipient strain for efficiently carrying out the method called “Red-driven integration” or “Red-mediated integration” (Datsenko, K A. and Wanner, B. L., 2000, Proc. Natl. Acad. Sci. USA., 97, 6640-6645) was constructed.
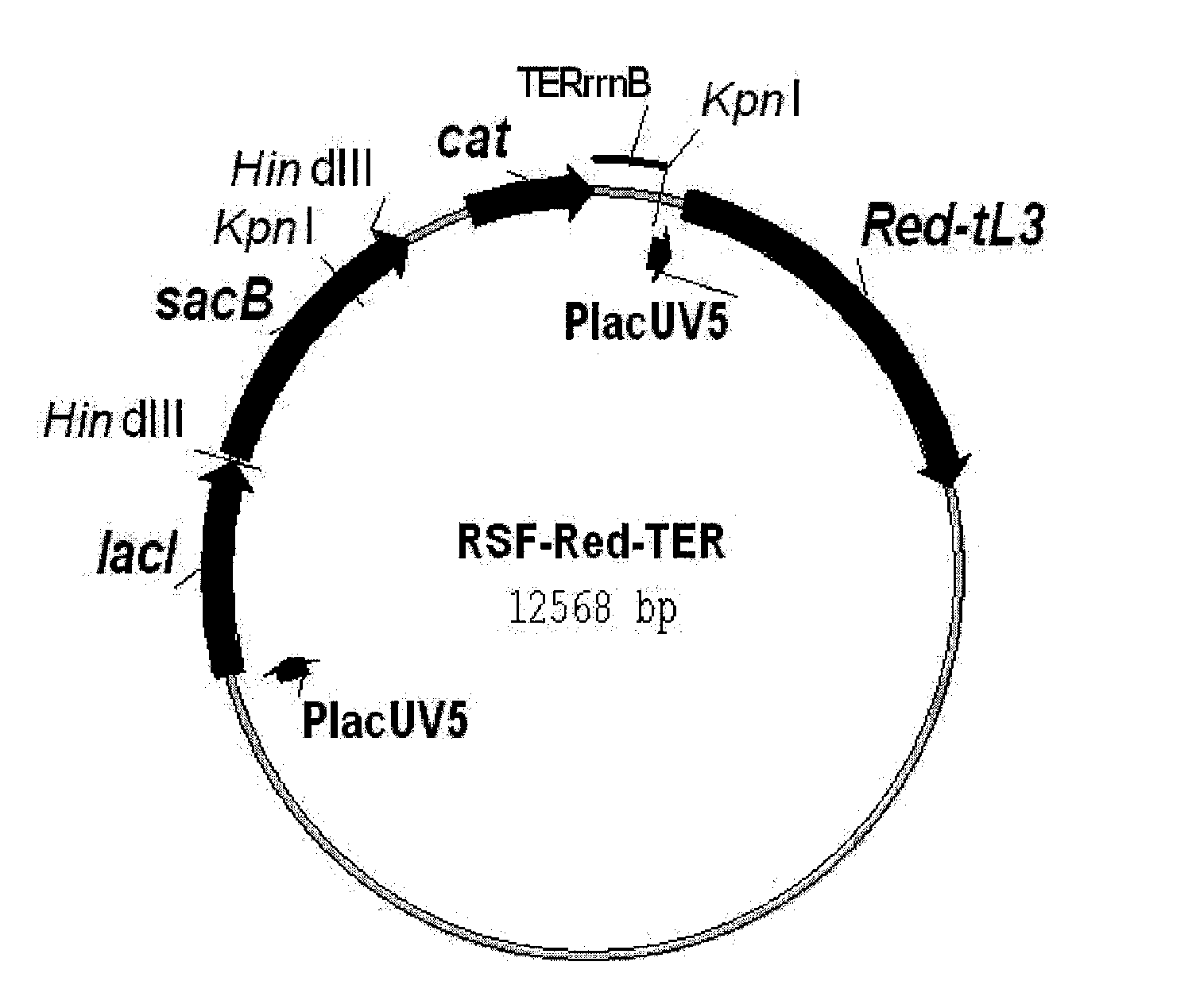
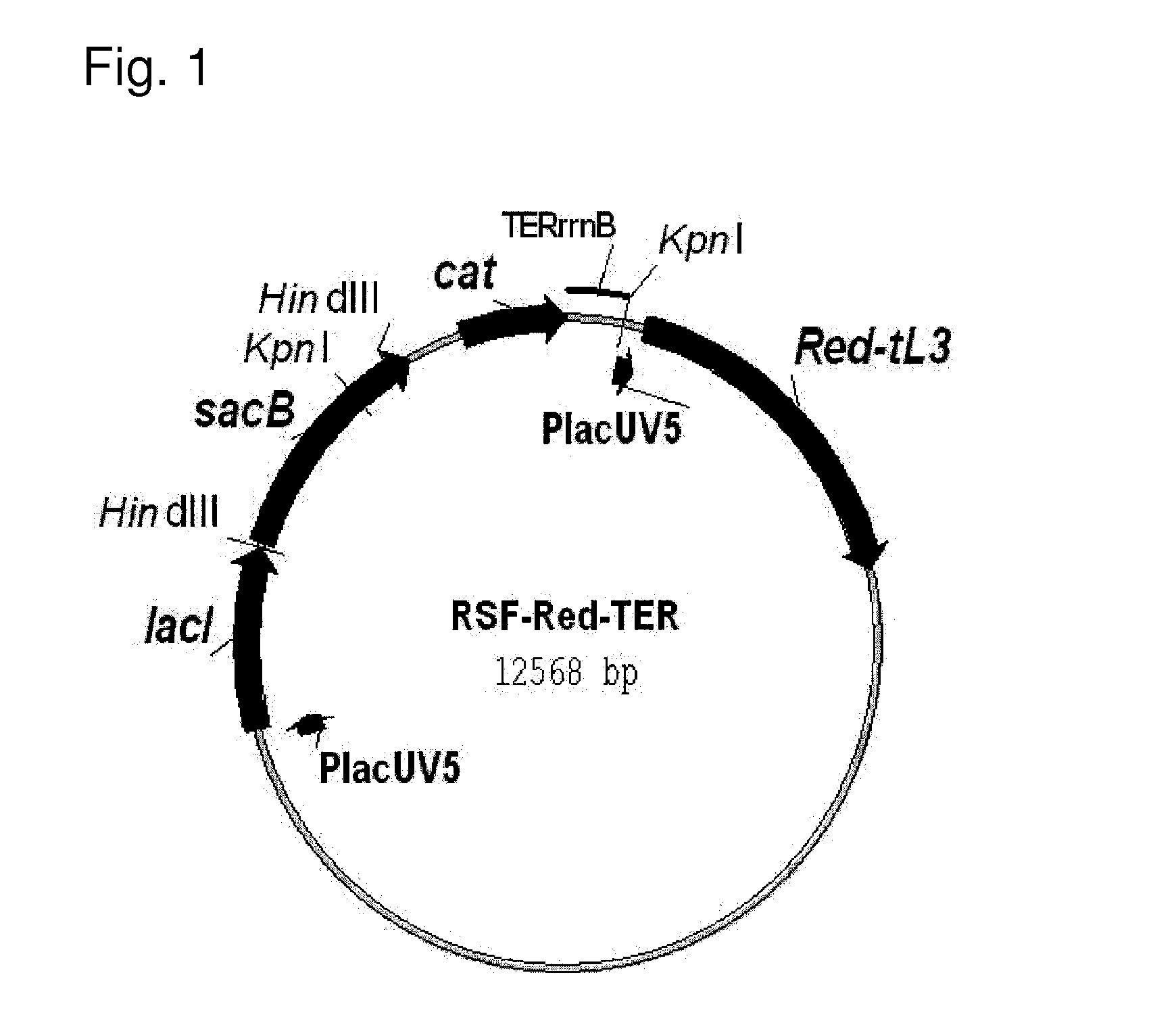
[0205]First, the novel helper plasmid RSF-Red-TER which expresses the gam, bet and exo genes of λ (“λ Red genes”) was constructed (FIG. 1). The details of this construction are described in Reference Example 2.
[0206]This plasmid can be used in a wide range of hosts having different genetic backgrounds. This is because 1) this plasmid has the replicon of the RSF1010 wide host spectrum plasmid (Scholz, et al., 1989, Gene, 75:271-288; Buchanan-Wollaston et al., 1987, Nature, 328:172-175), which is stably maintained by many types of gram negative and gram positive bacteria, and even plant cells, 2) the λ Red genes, gam, bet and exo, are un...
reference example 2
Construction of Helper Plasmid RSF-Red-TER
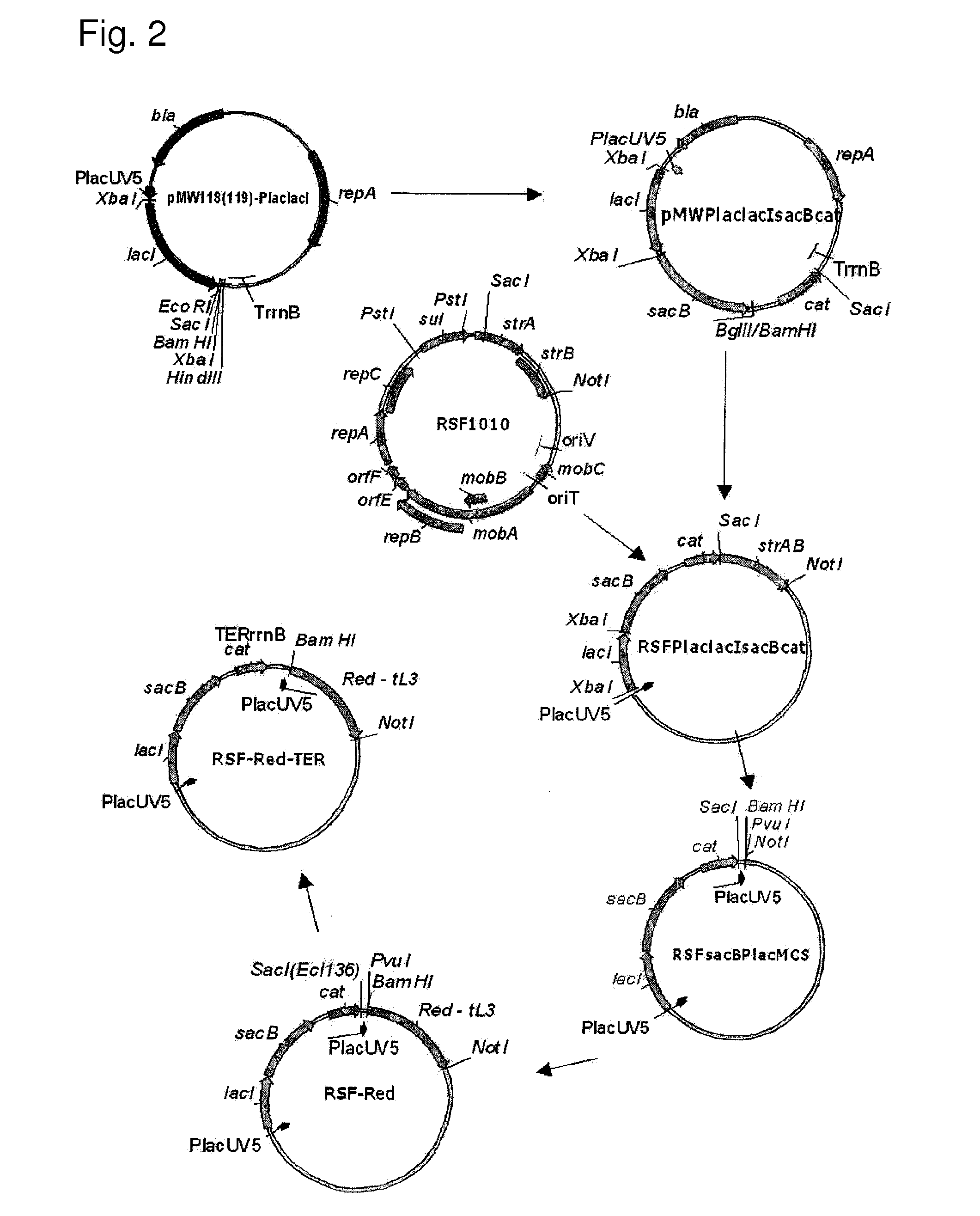
[0213]The scheme for constructing the helper plasmid RSF-Red-TER is shown in FIG. 2.
[0214]As the first step in the construction, an RSFsacBPlacMCS vector was designed. For this purpose, DNA fragments containing the cat gene of the pACYC184 plasmid and the structural region of the sacB gene of Bacillus subtilis were amplified by PCR using the oligonucleotides of SEQ ID NOS: 43 and 44, and 45 and 46, respectively. These oligonucleotides contained the BglII, Sad, XbaI and BamHI restriction enzyme sites in the 5′ end regions. These sites are required and convenient for further cloning. The obtained sacB fragment of 1.5 kb was cloned into the previously obtained pMW119-PlaclacI vector at the XbaI-BamHI site. This vector was constructed in the same manner as that described for the pMW118-PlaclacI vector (Skorokhodova, A. Y. et al, 2004, Biotekhnologiya (Rus), 5:3-21). However, this vector contained a polylinker moiety derived from pMW219 instead o...
reference example 3
Construction of the pMW118-(λattL-Kmr-λattR) Plasmid
[0217]The pMW118-(λattL-Kmr-λattR) plasmid was constructed from the pMW118-attL-Tc-attR (WO2005 / 010175) plasmid by replacing the tetracycline resistance marker gene with the kanamycin resistance gene of the pUC4K plasmid. For that purpose, the EcoRI-HindIII large fragment from pMW118-attL-Tc-attR was ligated to two fragments from the pUC4K plasmid: the HindIII-PstI fragment (676 bp) and EcoRI-HindIII fragment (585 bp). Basic pMW118-attL-Tc-attR was obtained by ligation of the following four fragments.
[0218]1) The BglII-EcoRI fragment (114 bp) including attL (SEQ ID NO: 55) which was obtained by PCR amplification of the region corresponding to attL of the Escherichia coli W3350 (containing λ prophage) chromosome using the primers P1 and P2 (SEQ ID NOS: 53 and 54) (these primers contained the subsidiary recognition sites for BglII and EcoRI).
[0219]2) The PstI-HindIII fragment (182 bp) including attR (SEQ ID NO: 58) which was obtained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com